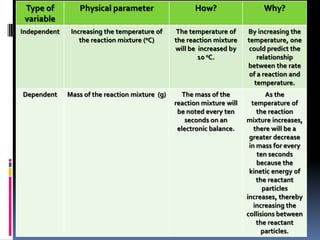
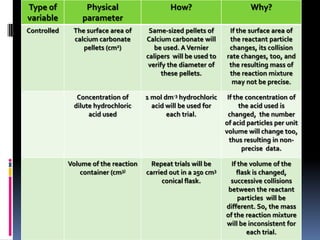
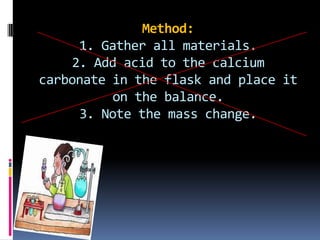
This document provides guidance on writing an eighth grade science lab report on how temperature affects the rate of a chemical reaction. It recommends writing a focused research question that specifies the independent variable (temperature from 40 to 80 degrees Celsius) and dependent variable (mass of reaction mixture in grams). The hypothesis should state that as temperature increases, the mass will decrease due to one of the products escaping as a gas. The method section describes the controlled variables and step-by-step process for conducting trials at different temperatures, recording data, and presenting it in a table.