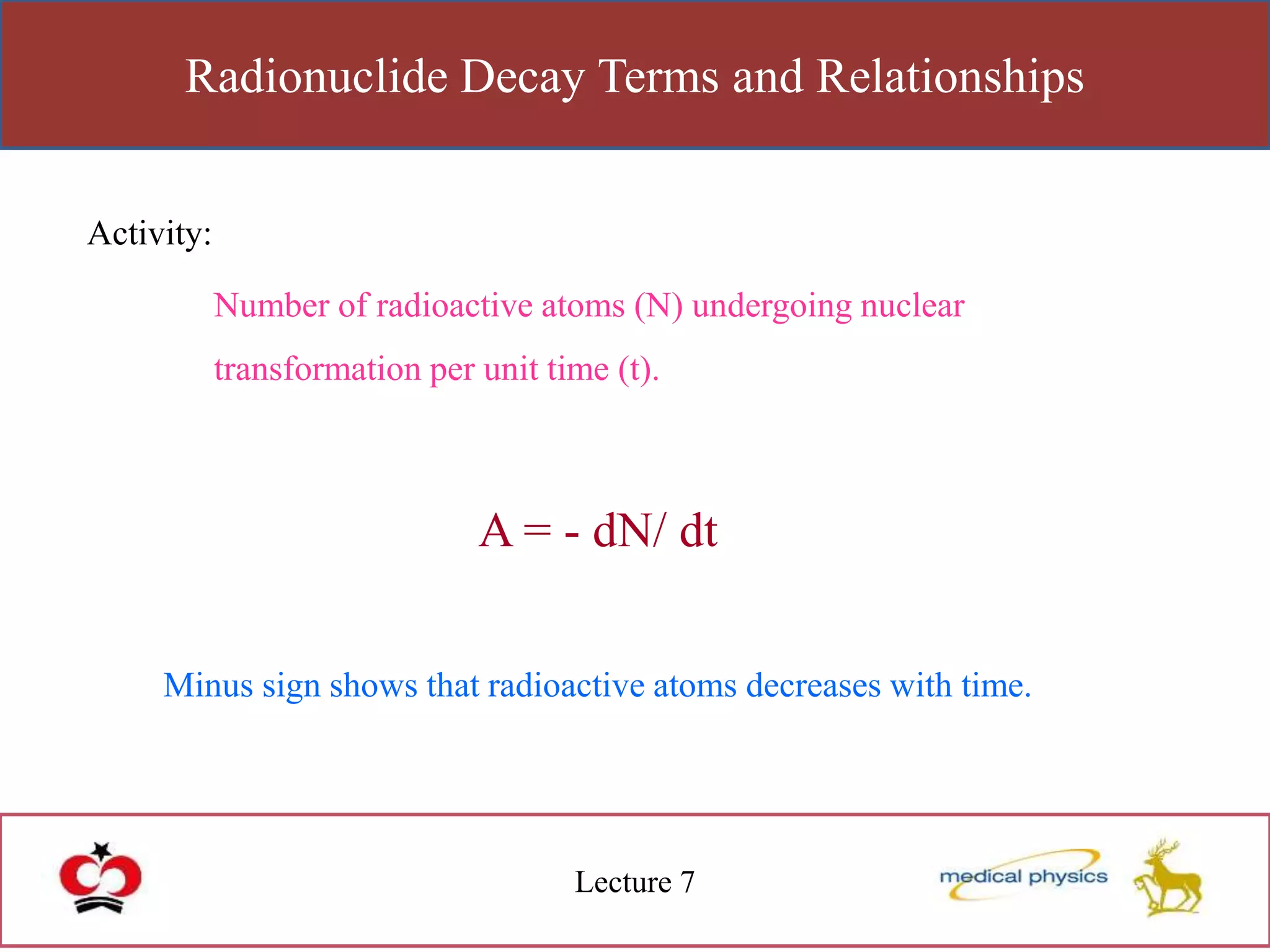
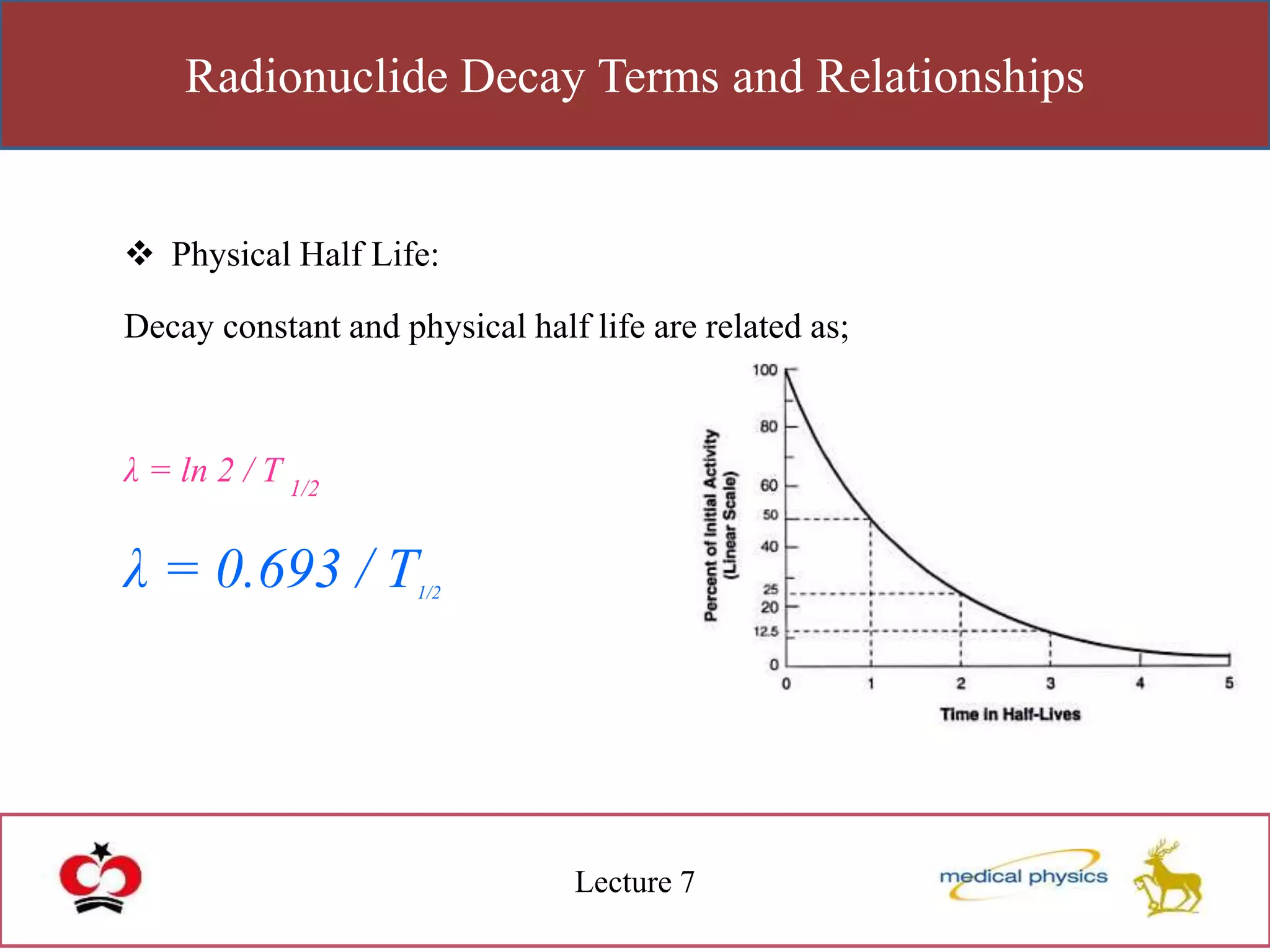
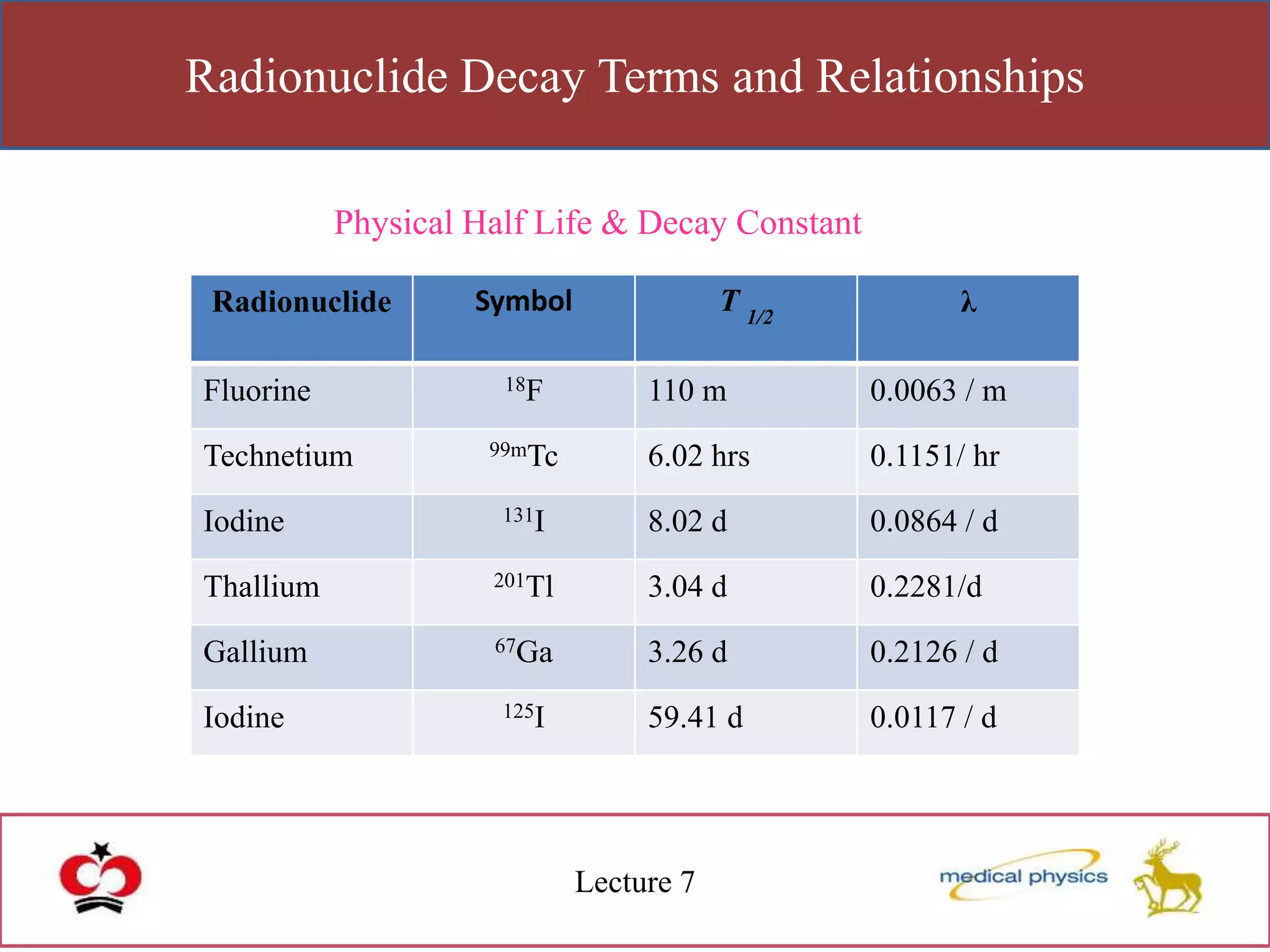
This lecture discusses the discovery of radioactivity by Henri Becquerel in 1896. Some key points:
1) Becquerel discovered that uranium salts would expose photographic plates even when not exposed to light, emitting "mysterious rays" that could pass through materials like x-rays.
2) Further experiments showed the rays were not dependent on any external source and the effect did not fade over time, challenging the idea of phosphorescence.
3) The discovery of radioactivity was an unexpected finding that burst onto the scientific scene. It showed that atoms of some elements can spontaneously emit radiation through nuclear decay.