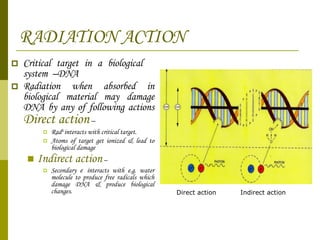
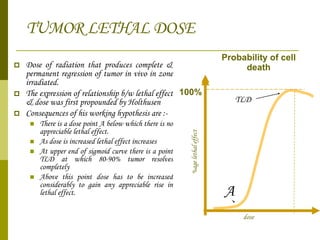
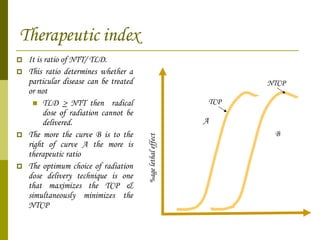
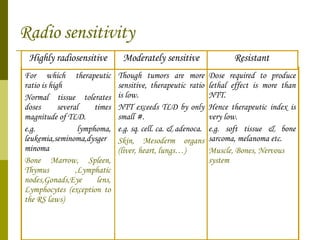
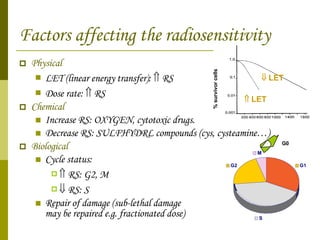
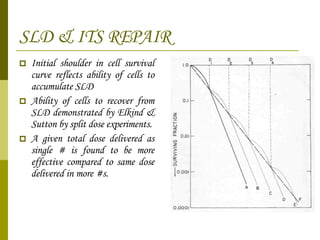
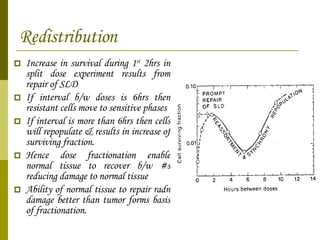
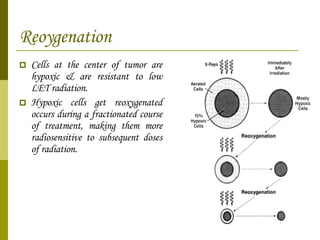
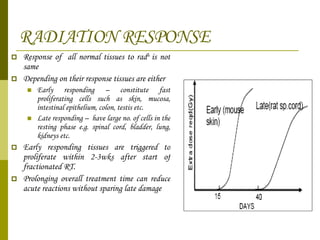
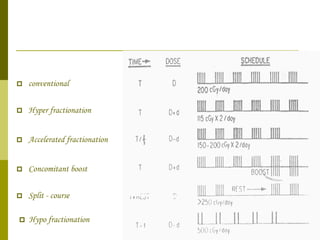
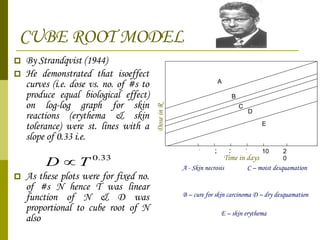
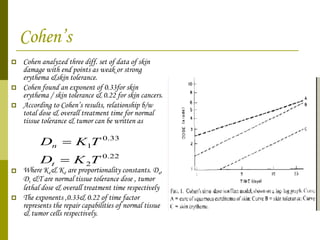
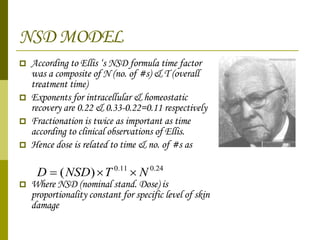
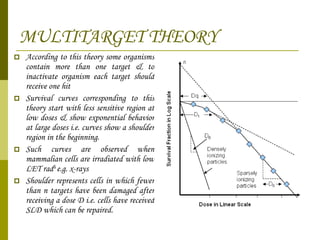
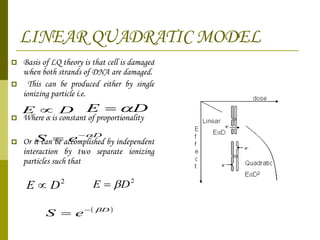
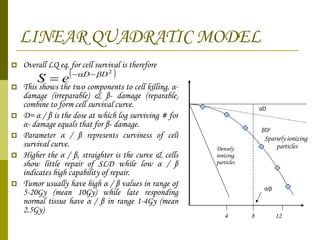
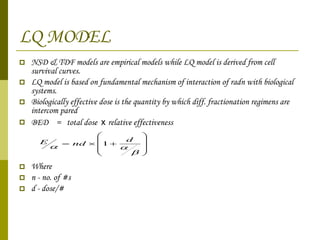
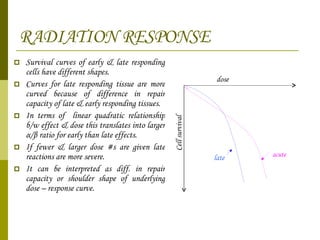
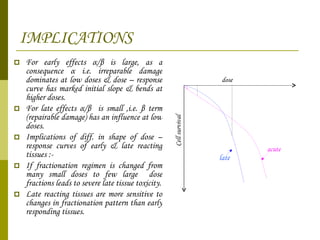
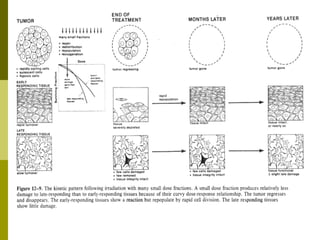
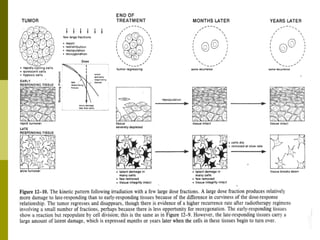
The document discusses key concepts in radiation oncology including dose fractionation, tumor lethal dose, normal tissue tolerance, and factors that affect radiosensitivity. Fractionating the total radiation dose into smaller daily doses allows time for repair of sublethal damage in normal tissues, improving the therapeutic ratio by reducing side effects while still effectively treating the tumor.