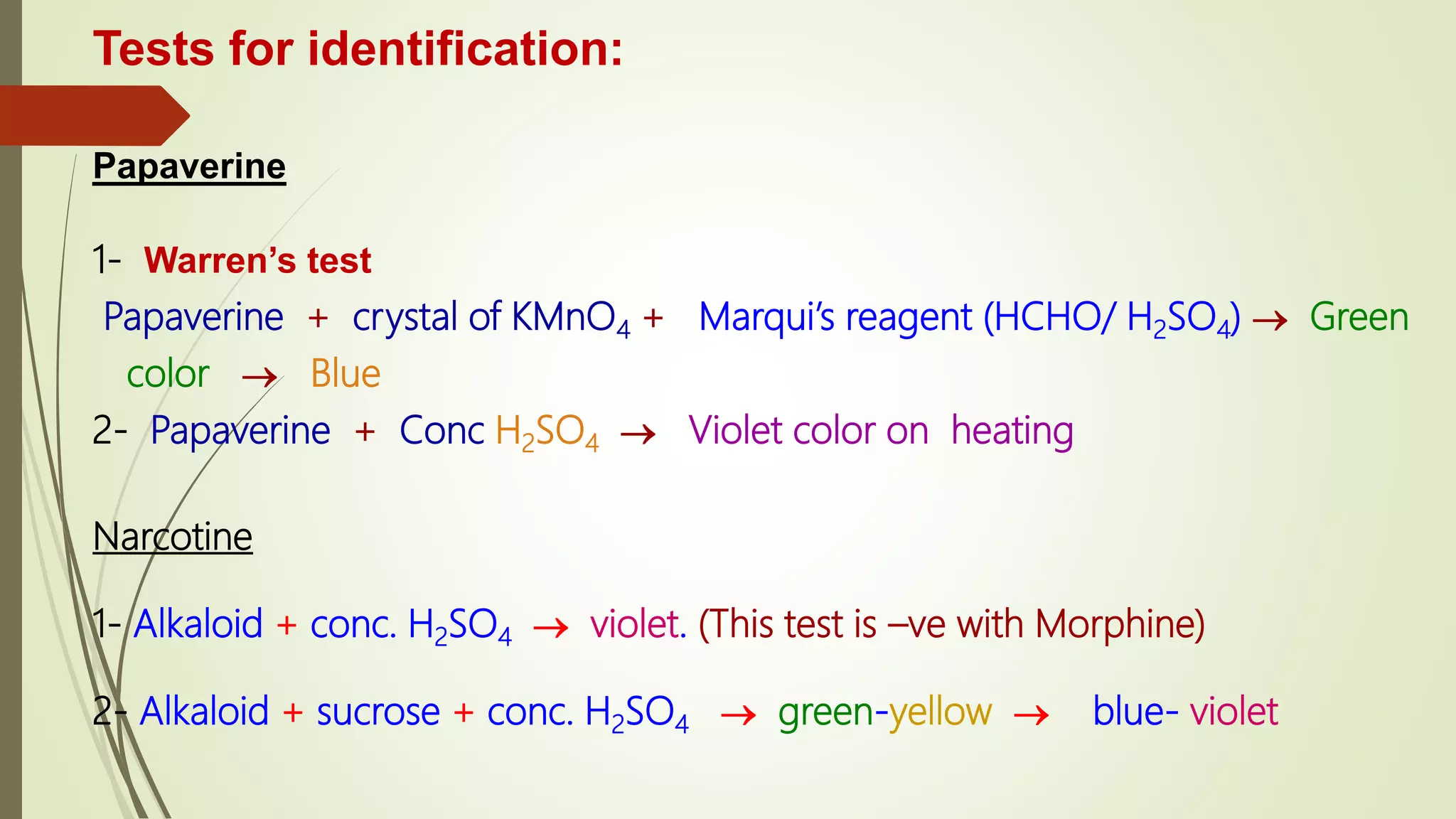
This document discusses opium alkaloids, derived from the opium poppy, their historical significance, and their medicinal properties. It details various alkaloids such as morphine, codeine, and papaverine, including their chemical characteristics, medical uses, and the processes involved in their extraction and identification. The text also addresses the implications of opium addiction, its historical context, and the ongoing development of semi-synthetic and synthetic derivatives for medical purposes.












![OpiumAlkaloid of the Benzylisoquinoline gruop
◘ Papaverine and Narcotine are very different from the morphine, codeine, thebaine group of
alkaloids (Morphinans).
◘ Papaverine and Narcotine (Noscapine) are very weak bases, their solutions are neutral to litmus
paper.
◘ The free bases can be extracted from their acid solution [unstable salts] with CHCl3.
◘ They are derived biosynthetically from Tyrosine amino acid.
N
O
O
H
OCH3
OCH3
O
H
CH3
O
OCH3
Narcotine (Noscapine)
N
OCH3
OCH3
H3CO
H3CO
Papaverine](https://image.slidesharecdn.com/opiumalkaloidsandderivatives-180403122549/75/Opium-alkaloids-and-derivatives-13-2048.jpg)