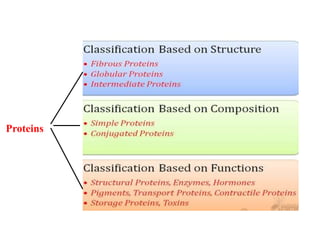
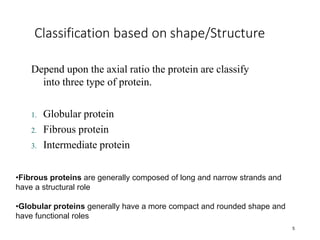
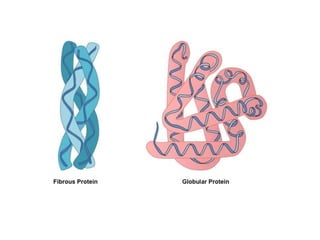
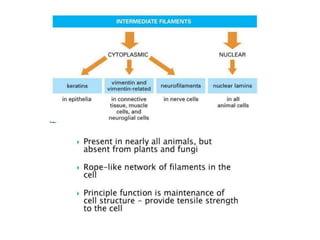
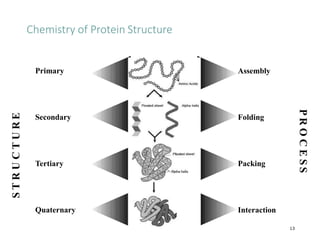
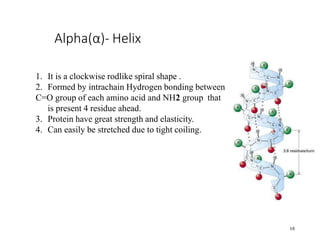
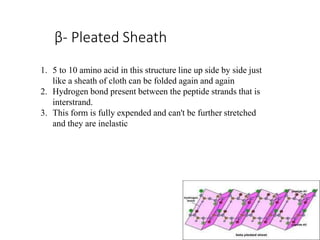
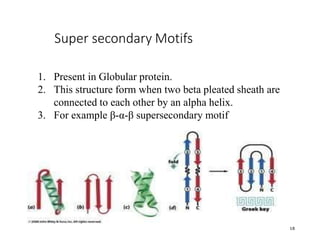
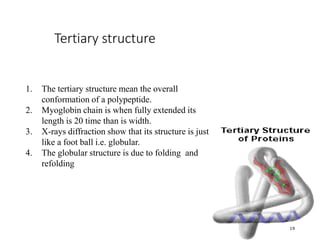
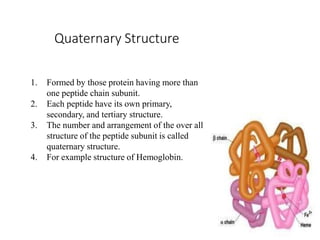
This document discusses protein classification and structure. It begins by defining proteins and noting that there are 22 standard amino acids that make up all proteins. Proteins are then classified based on their shape into globular, fibrous, and intermediate proteins. Globular proteins are more compact and spherical while fibrous proteins are long strands used for structure. The document also discusses protein structure at the primary, secondary, tertiary, and quaternary levels. Primary structure is the amino acid sequence, secondary involves coiling into alpha helices or beta sheets, tertiary is the protein's 3D shape from folding, and quaternary involves interactions of multiple protein subunits. Common structural proteins like collagen and keratin are also mentioned.