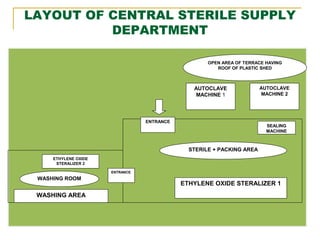
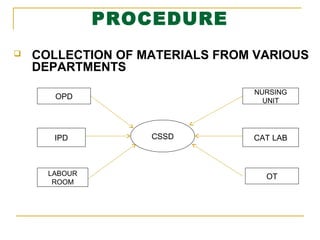
This document provides information about the Central Sterile Supply Department (CSSD) at Shri Krishna Hrudayalaya & Critical Care Hospital in Nagpur, India. It discusses the aims, objectives and methodology of the internship project. It introduces the CSSD and describes its functions like processing, sterilizing and distributing sterile equipment and supplies to different hospital departments. It outlines the various areas within the CSSD including receiving, cleaning, packing, sterilizing, storage and distribution. The goal of the CSSD is to reliably provide sterilized items when and where needed in the hospital in a cost-effective manner.










![INTRODUCTION OF CSSD
ENSURING A HIGH STANDARD OF STERILIZATION AND DISINFECTION TO MINIMIZE THE INCIDENCE OF
HOSPITAL INFECTION HAS BEEN UPPERMOST IN THE MINDS OF CLINICIANS AS WELL AS HOSPITAL
ADMINISTRATORS. STANDARDIZATION OF SURGICAL DRESSINGS, AND CENTRALIZING ALL SURGICAL
SUPPLIES FROM ONE POINT OF ORIGIN WERE NECESSITATED DURING THE SECOND WORD WAR BECAUSE
OF THE REQUIREMENT OF A LARGE NUMBER OF CASUALTIES IN DIFFERENT THEATERS OF WAR. THE
CONCEPT OF A STERILE SUPPLY ORGANIZATION, IN THE FORM OF AN INDEPENDENT UNIT OR ONE
ATTACHED TO LARGE BASE HOSPITALS CAME INTO BEING. STERILIZATION ACTIVITIES IN A HOSPITAL ARE
BETTER CENTRALIZED IN ONE SINGLE DEPARTMENT FOR EFFICIENCY AND EFFECTIVENESS. THIS
DEPARTMENT, CALLED THE CENTRAL STERILE SUPPLY DEPARTMENT (CSSD) BECOMES RESPONSIBLE FOR
PROCESSING, STERILIZING AND DISPENSING OF ALMOST ALL ITEMS OF STERILE EQUIPMENT, SETS AND
DRESSINGS IN THE HOSPITAL. CENTRALIZATION OF STERILIZING ACTIVITIES IN ONE DEPARTMENT HAS
RESULTED IN MANY ADVANTAGES, THE CHIEF AMONG THESE ARE (I) IMPROVED EFFICIENCY, (II) STERILE
SUPPLIES AVAILABLE AT ALL TIMES OF THE DAY OR NIGHT, (III) ECONOMY OF TRAINED MANPOWER, (IV)
STERILIZATION SAFETY AND (V) QUALITY CONTROL.
THE OBJECTIVE OF ESTABLISHING A CENTRAL STERILE SUPPLY DEPARTMENT IS TO MAKE RELIABLY
STERILIZED ARTICLES AVAILABLE AT THE REQUIRED TIME AND PLACE FOR ANY AGREED PURPOSE IN THE
HOSPITAL AS ECONOMICALLY AS POSSIBLE, HAVING REGARD TO THE NEED TO CONSERVE THE TIME OF
USERS [ESPECIALLY DOCTORS AND NURSES]. THE STERILE SUPPLY DEPARTMENT WITHIN A HOSPITAL
RECEIVES STORES, STERILIZES AND DISTRIBUTES TO ALL DEPARTMENTS INCLUDING THE WARDS,
OUTPATIENT DEPARTMENT [OPD] AND OTHER SPECIAL UNITS SUCH AS OPERATING THEATRE [OT]. MAJOR
RESPONSIBILITIES OF CSSD INCLUDE PROCESSING AND STERILIZATION OF SYRINGES, RUBBER GOODS
[CATHETERS, TUBING], SURGICAL INSTRUMENTS, TREATMENT TRAYS AND SETS, DRESSINGS ETC. IT IS
RESPONSIBLE FOR ECONOMIC AND EFFECTIVE UTILIZATION OF EQUIPMENT RESOURCES OF THE HOSPITAL
UNDER CONTROLLED SUPERVISION.
THE CSSD ALSO AIMS AT ASSUMING TOTAL RESPONSIBILITY FOR PROCESSING HOSPITAL ITEMS THEREBY
ASSURING THAT ALL OF THEM RECEIVE THE SAME DEGREE OF CLEANING AND STERILIZATION. IT ALSO
CONTRIBUTES TO THE EDUCATIONAL PROGRAM WITHIN THE HOSPITAL RELATING TO INFECTION CONTROL
AND DEVELOPS A COST-EFFECTIVE PROGRAM BY COST ANALYSIS OF PERSONNEL, SUPPLIES AND
EQUIPMENT.
](https://image.slidesharecdn.com/projectoncssd-130625015919-phpapp01/85/Project-on-cssd-14-320.jpg)