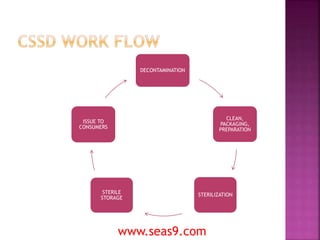
The Central Sterile Supply Department (CSSD) processes reusable medical devices for hospitals by cleaning, disinfecting, packaging, and sterilizing items. The CSSD area is divided into four sections - a dirty area for decontamination, a clean area for packaging and preparation, a linen packaging area, and a sterile storage area. The centralized CSSD model ensures staff are trained specifically for processing devices, the department is properly equipped, and quality control is maintained, improving safety.