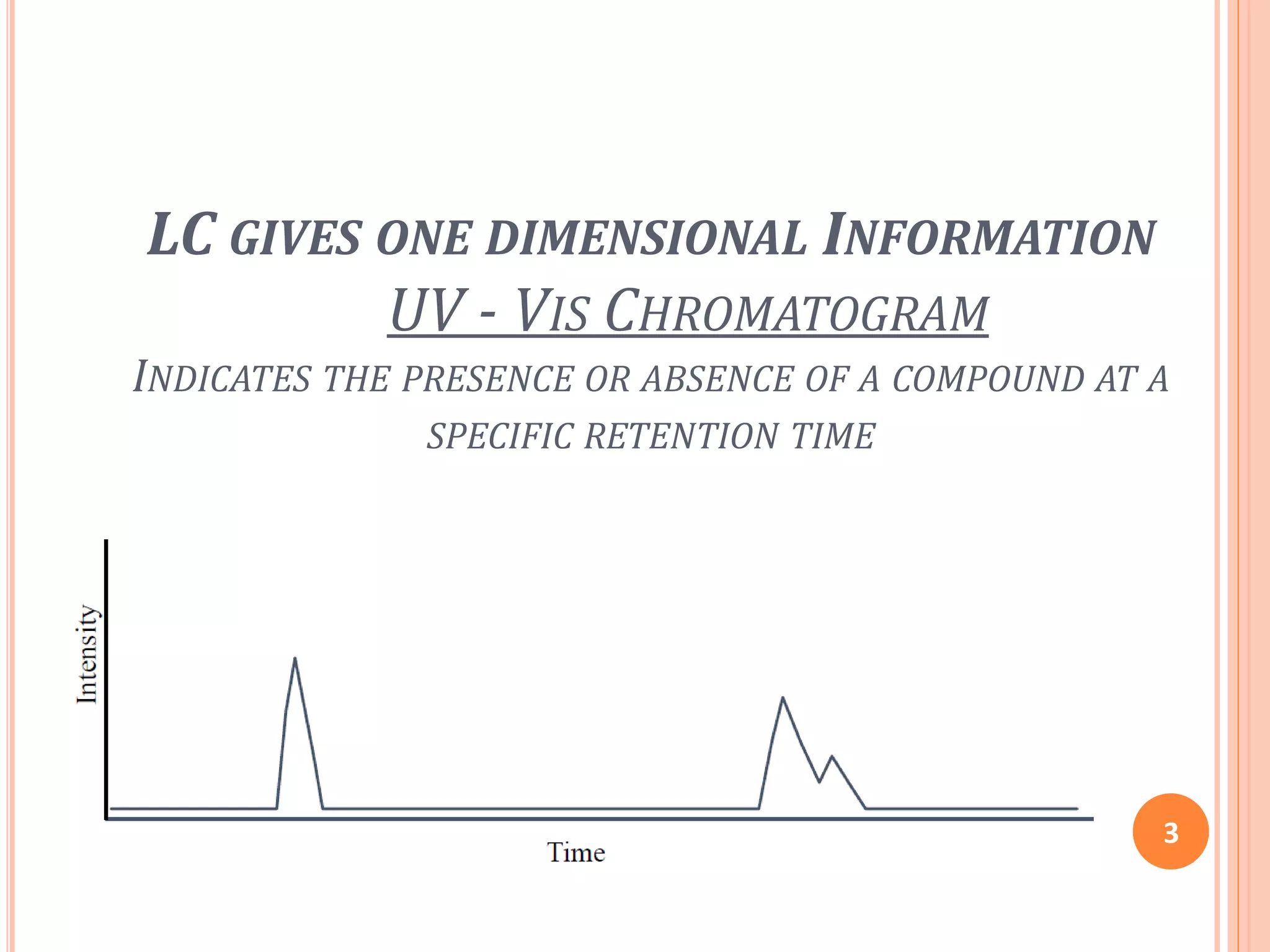
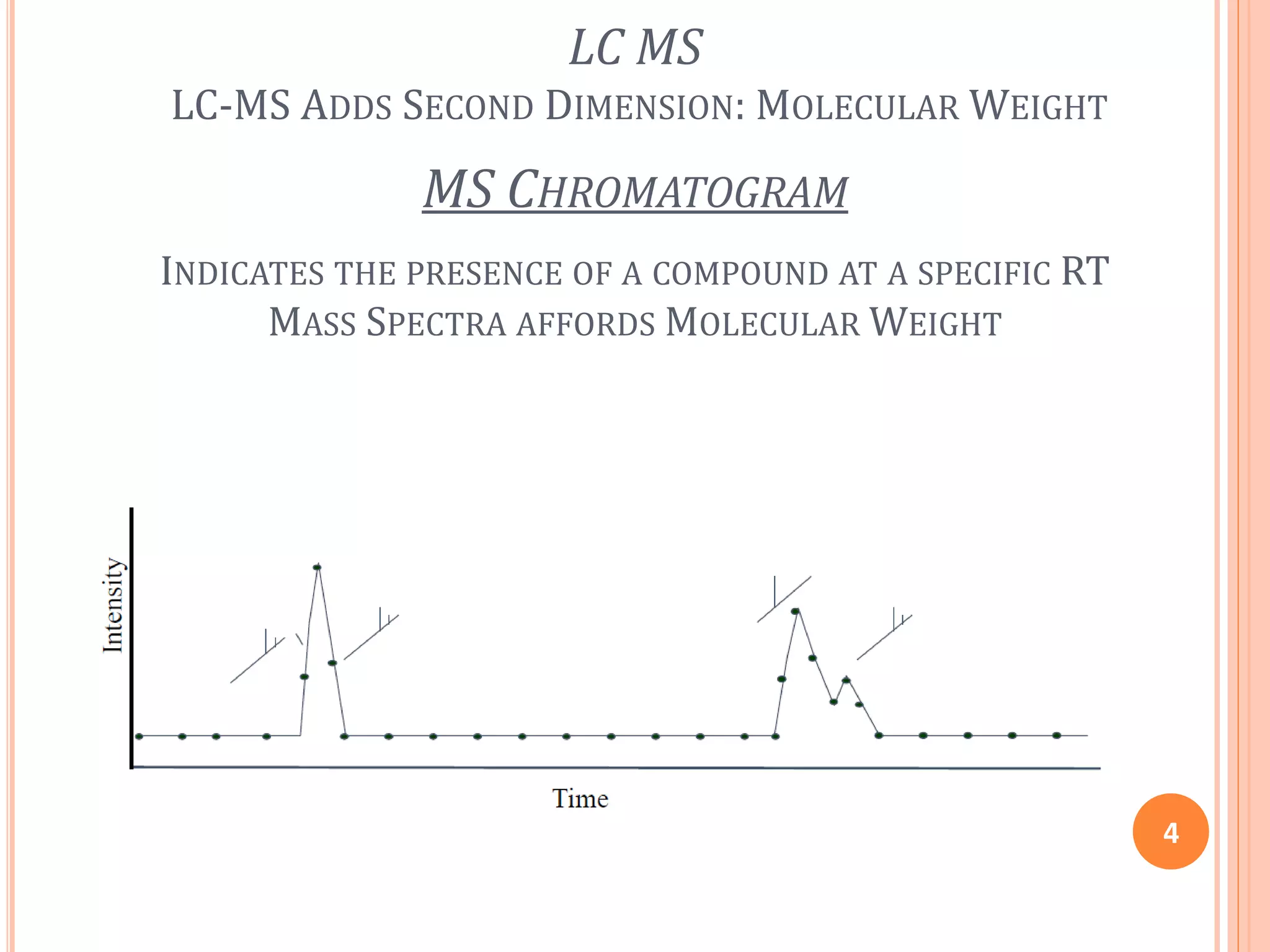
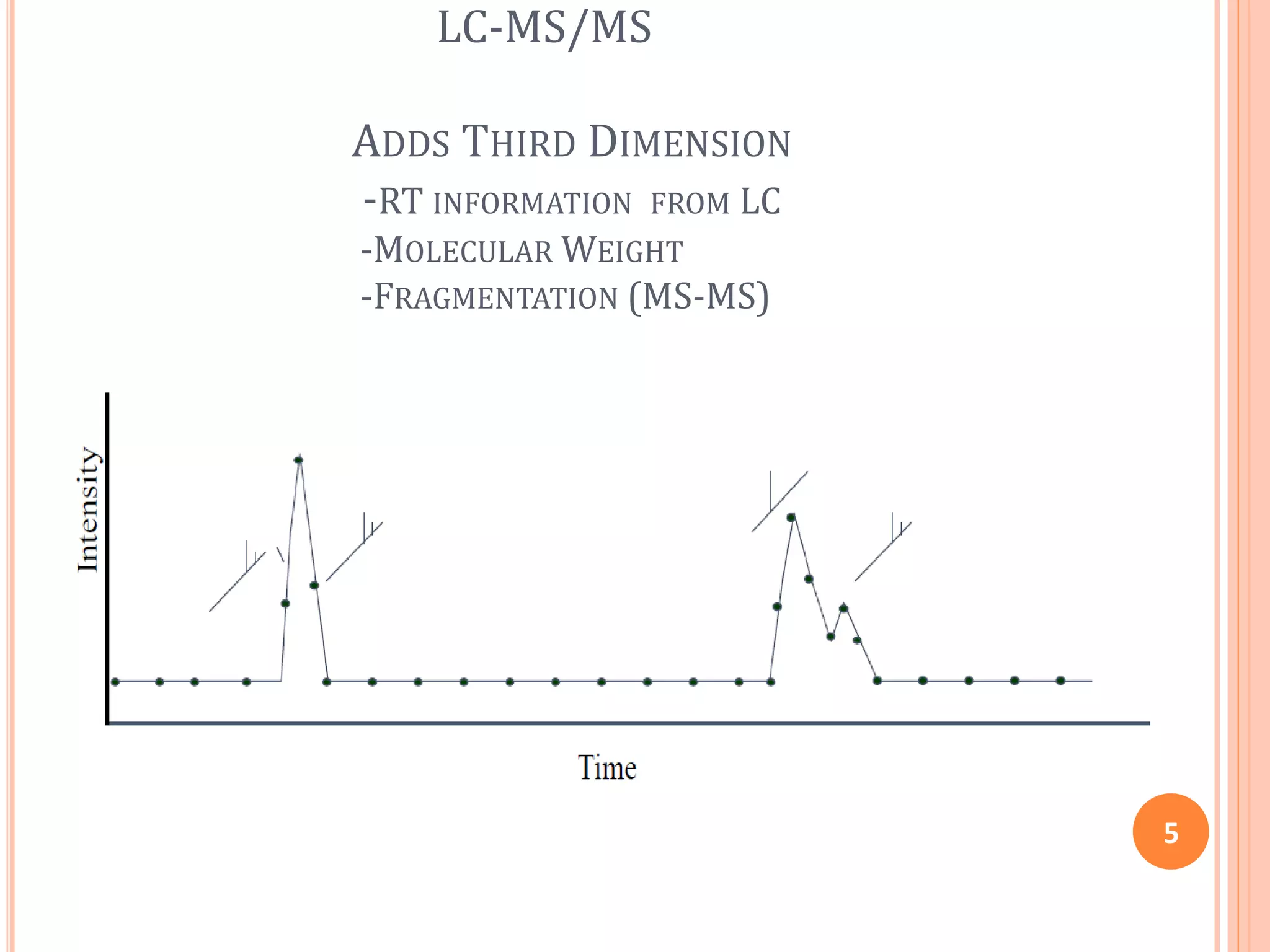
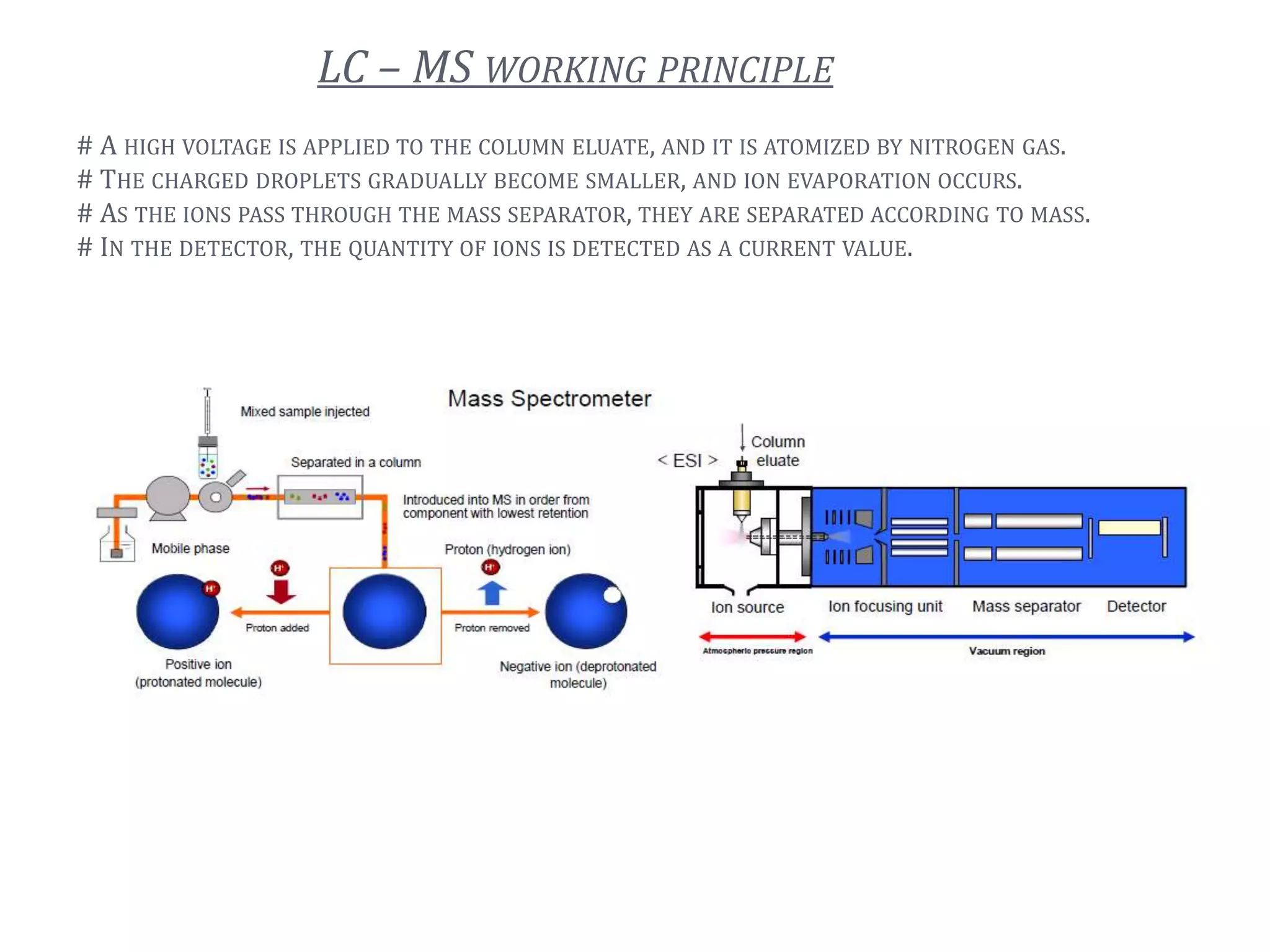
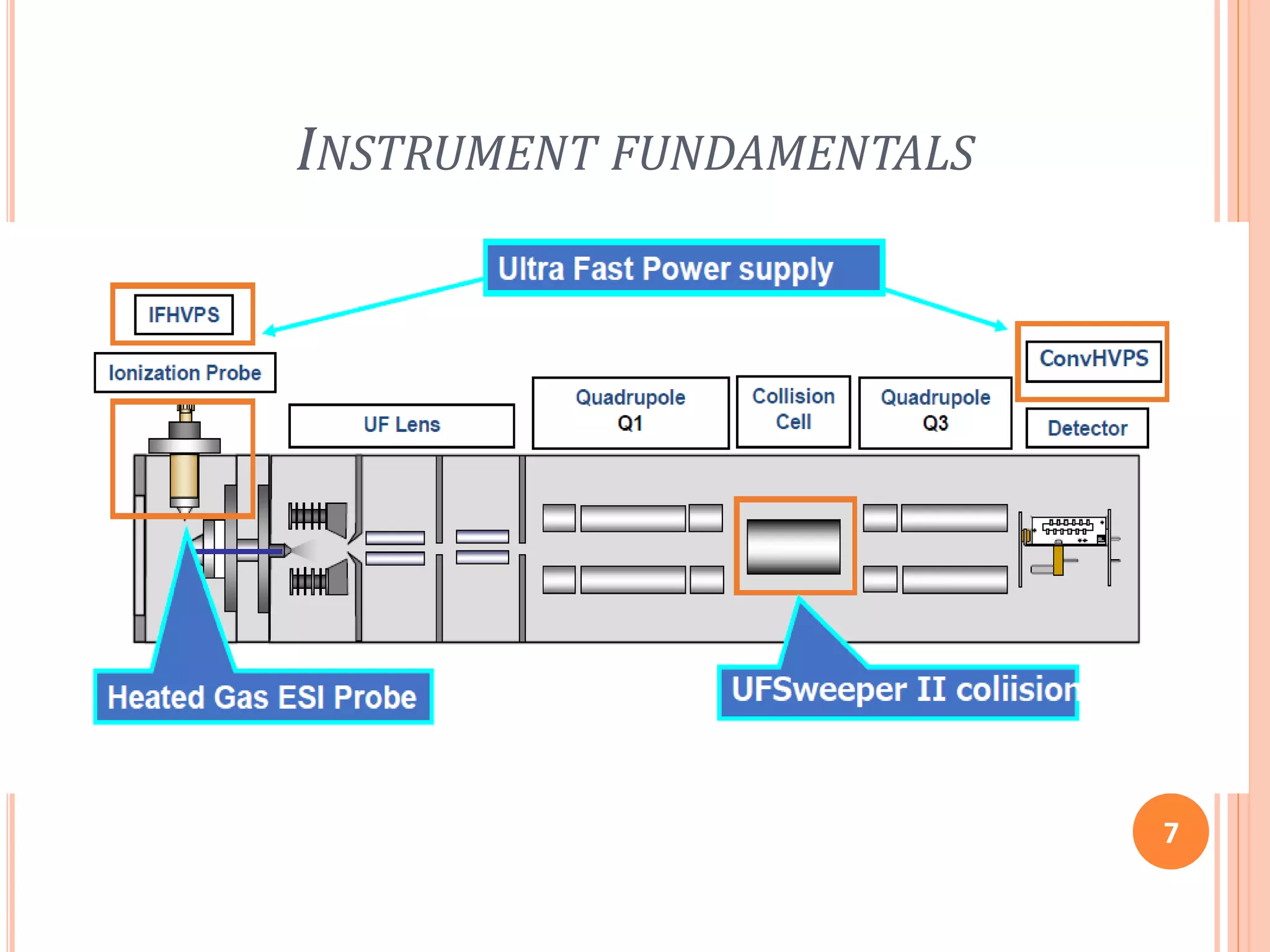
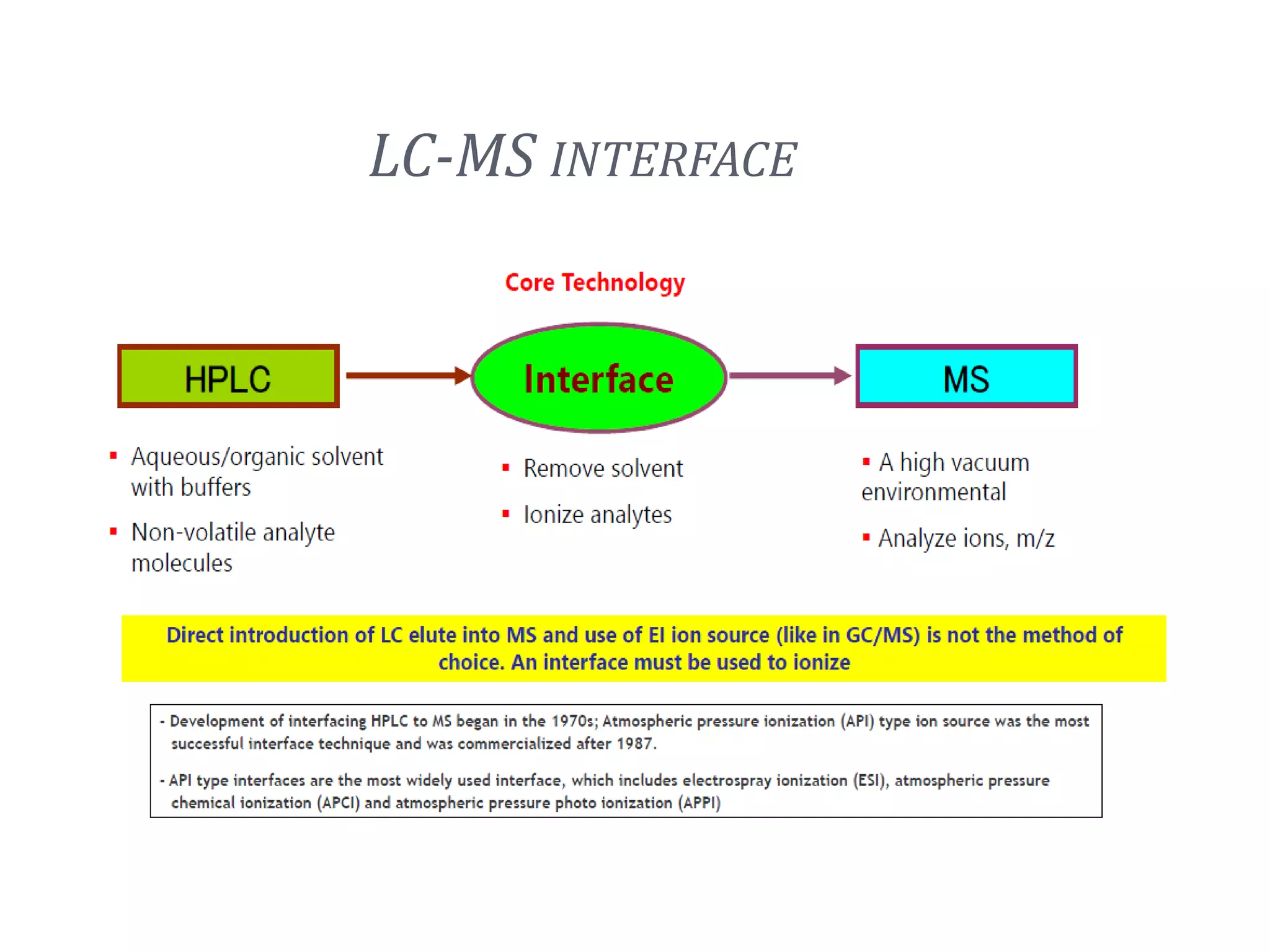
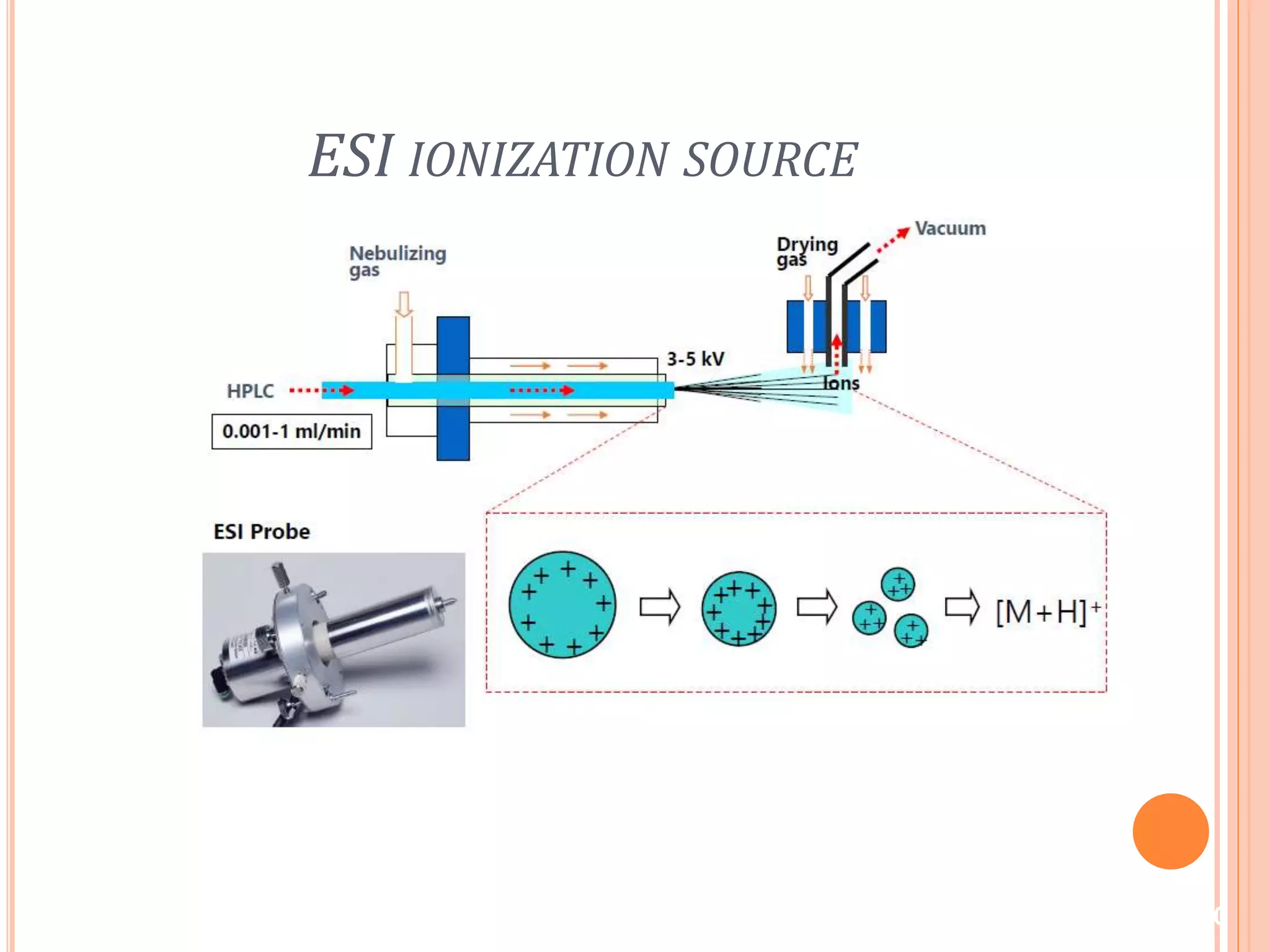
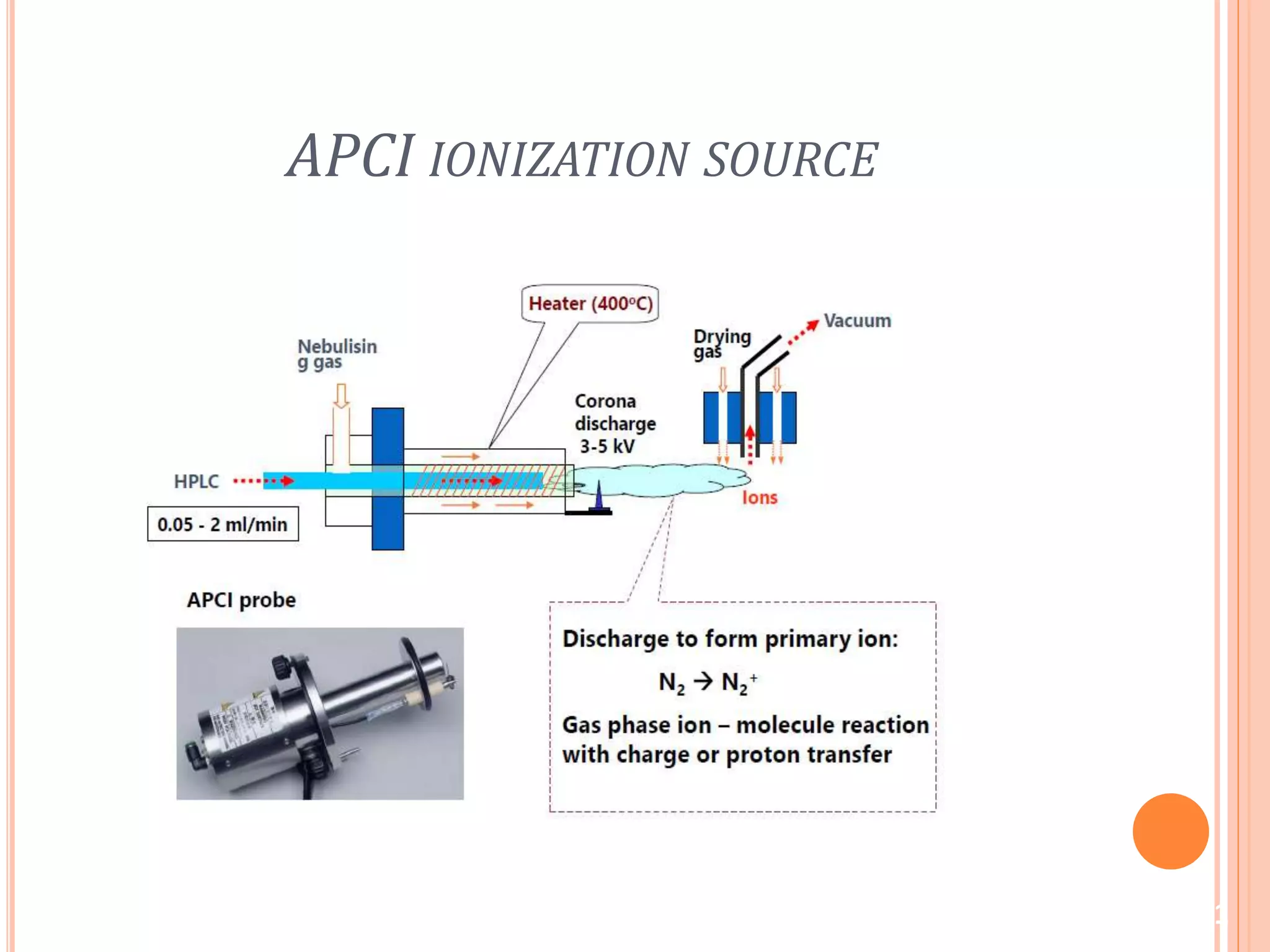
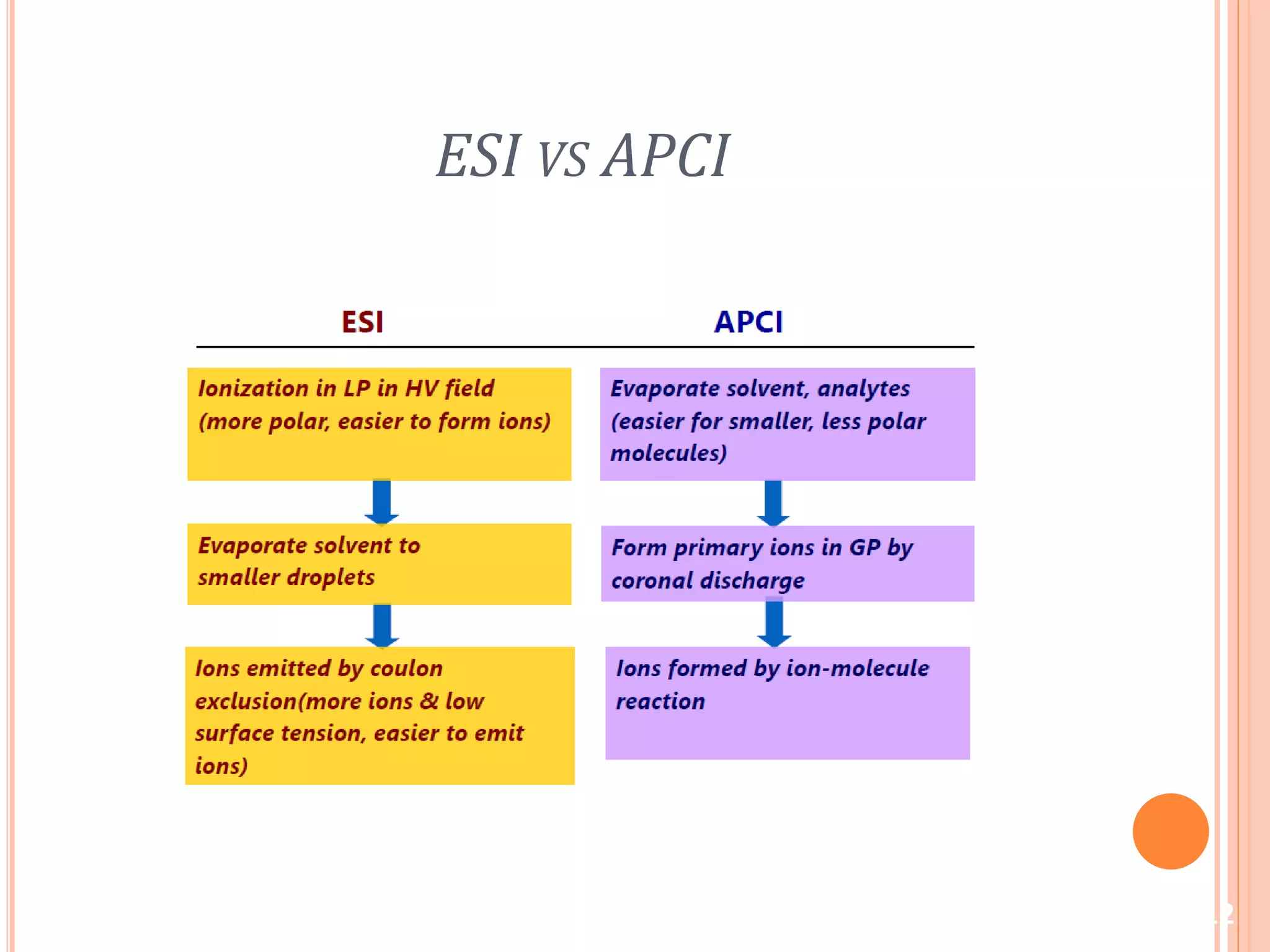
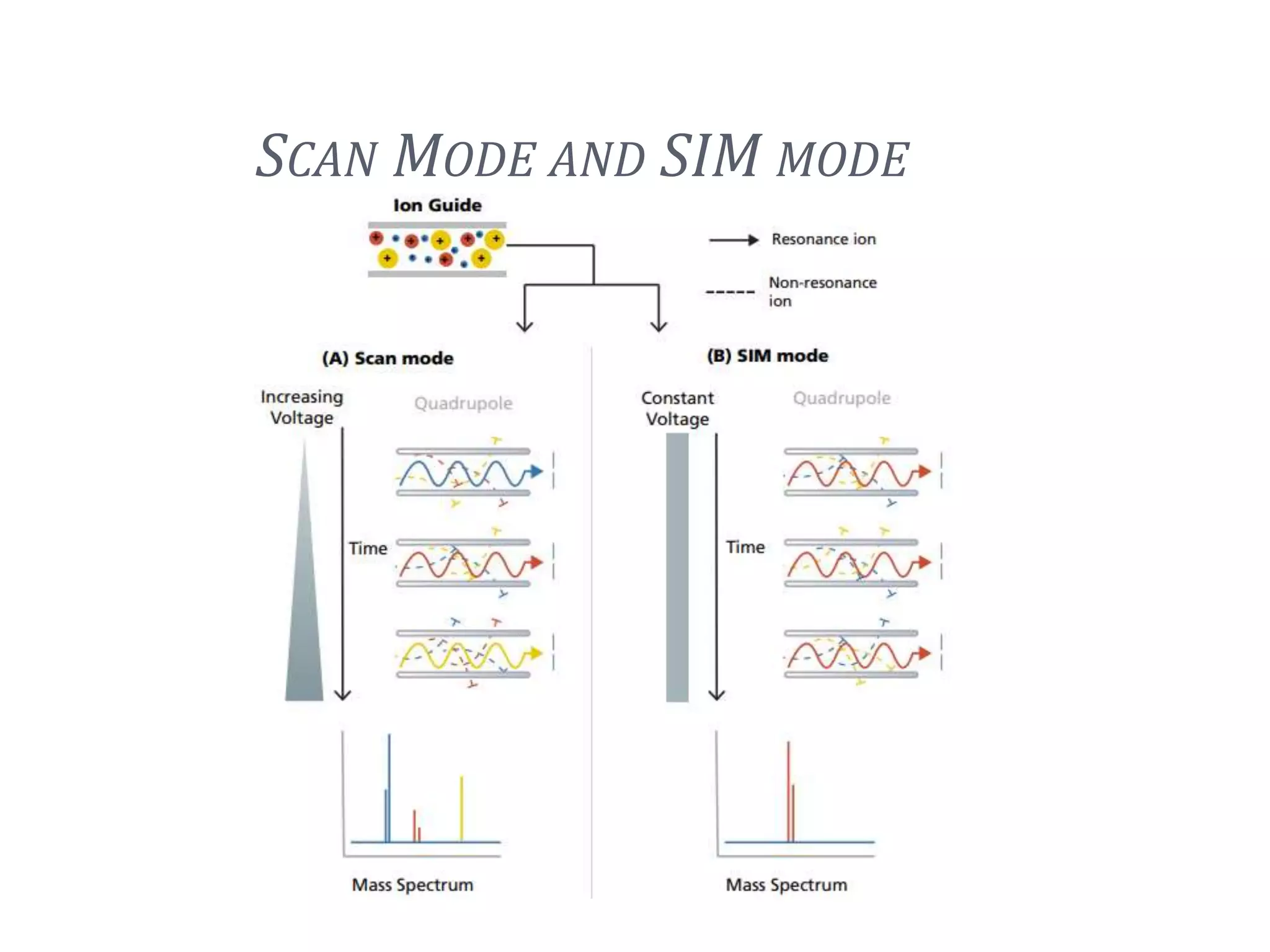
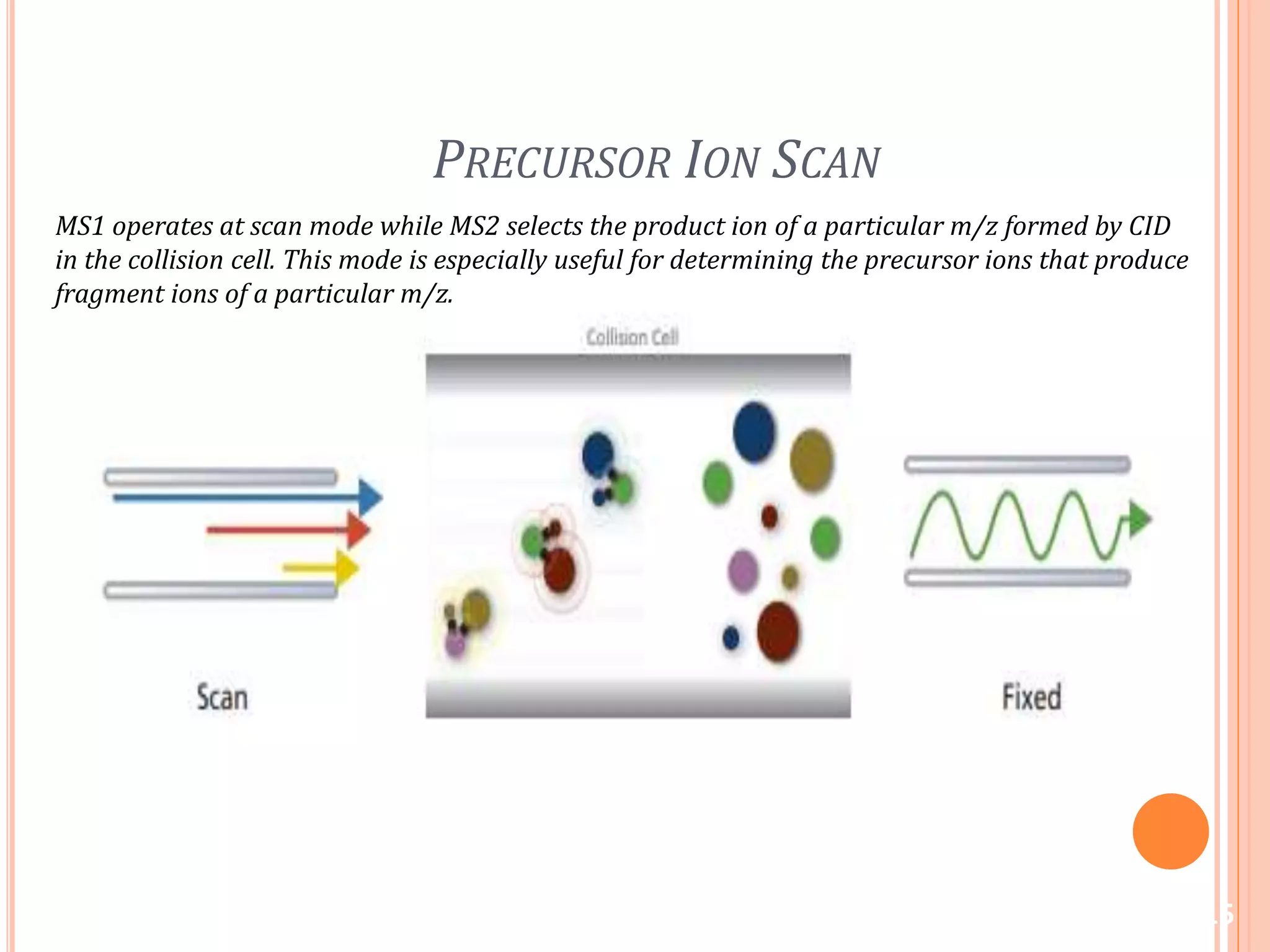
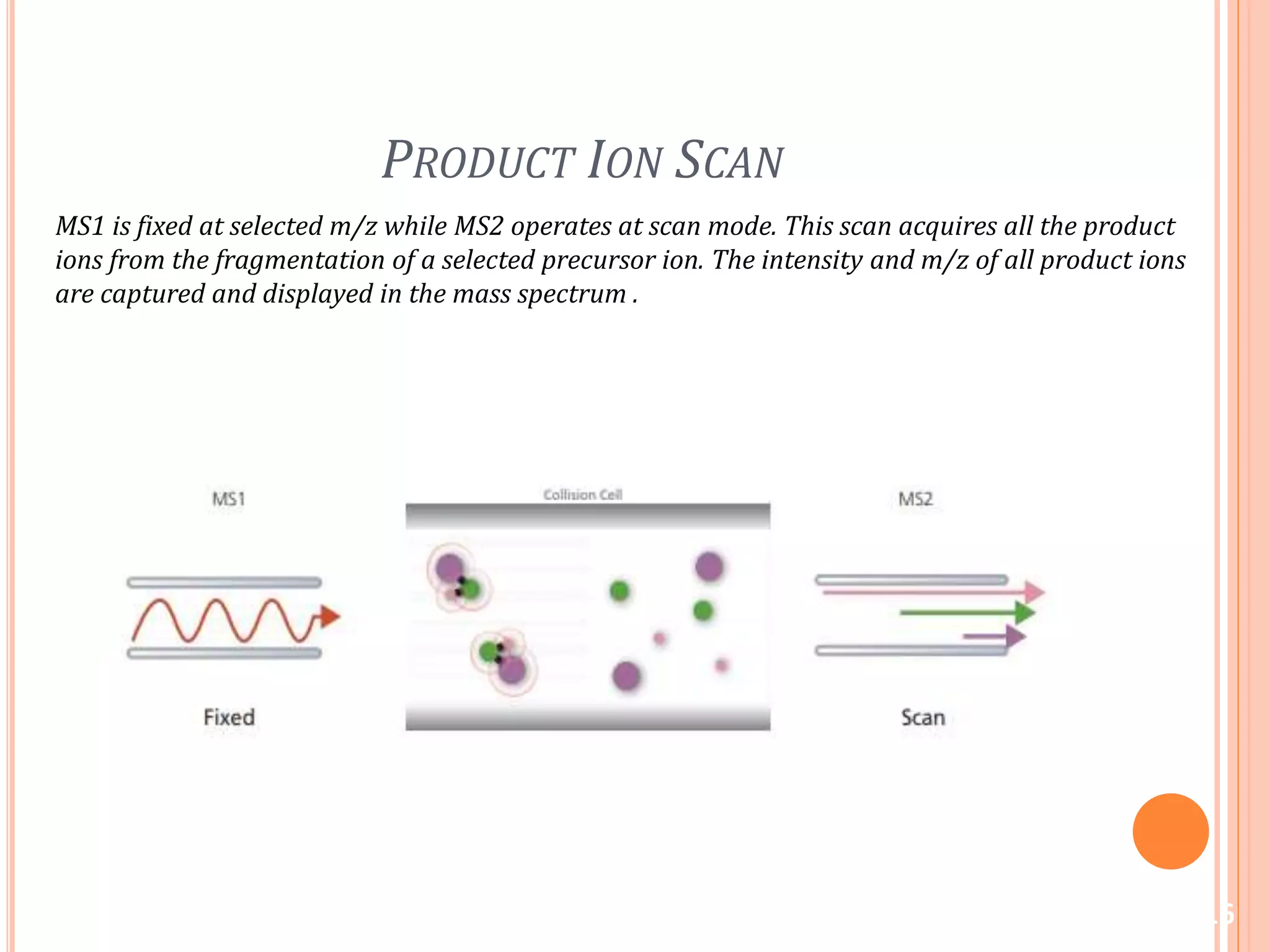
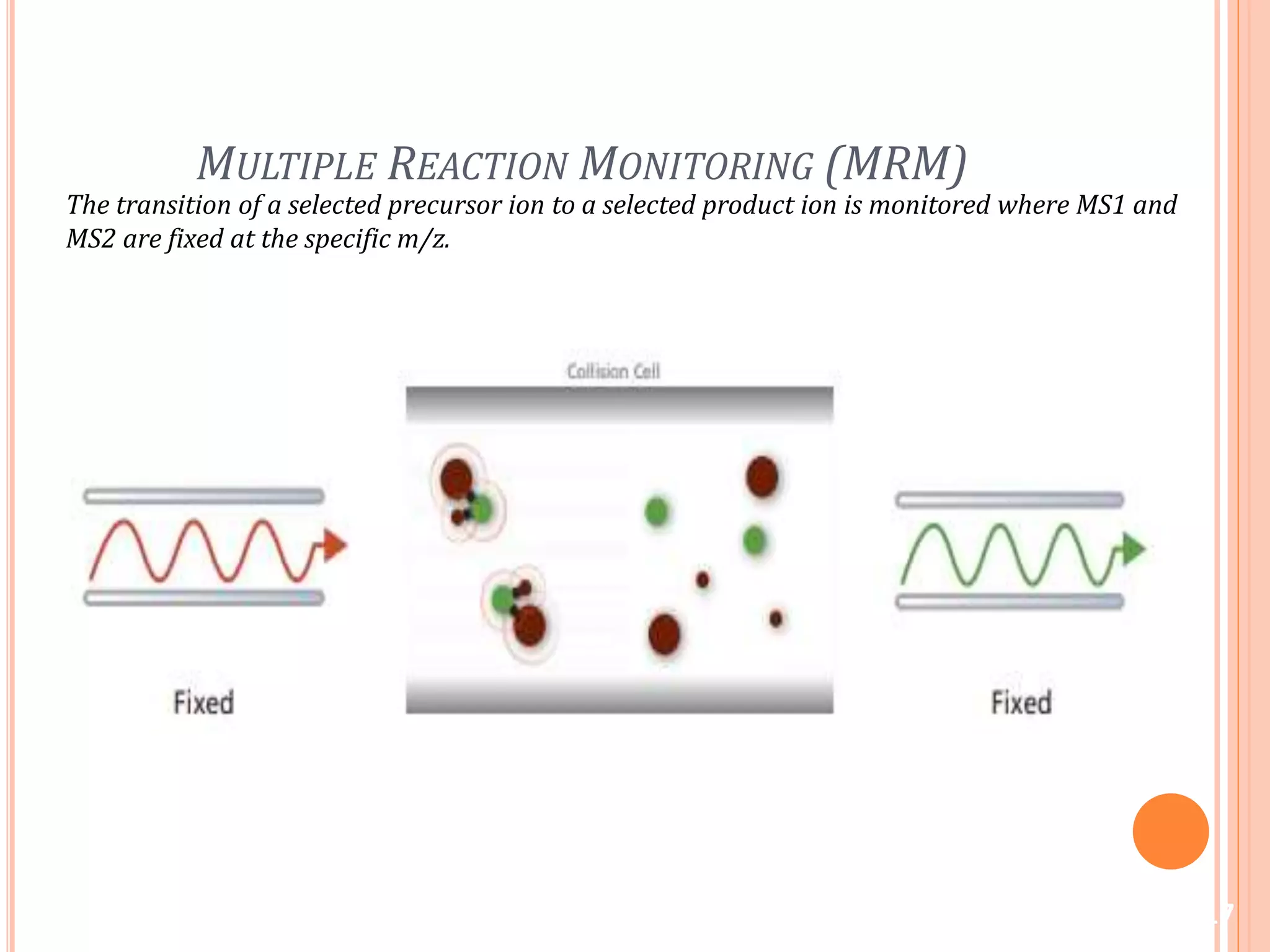
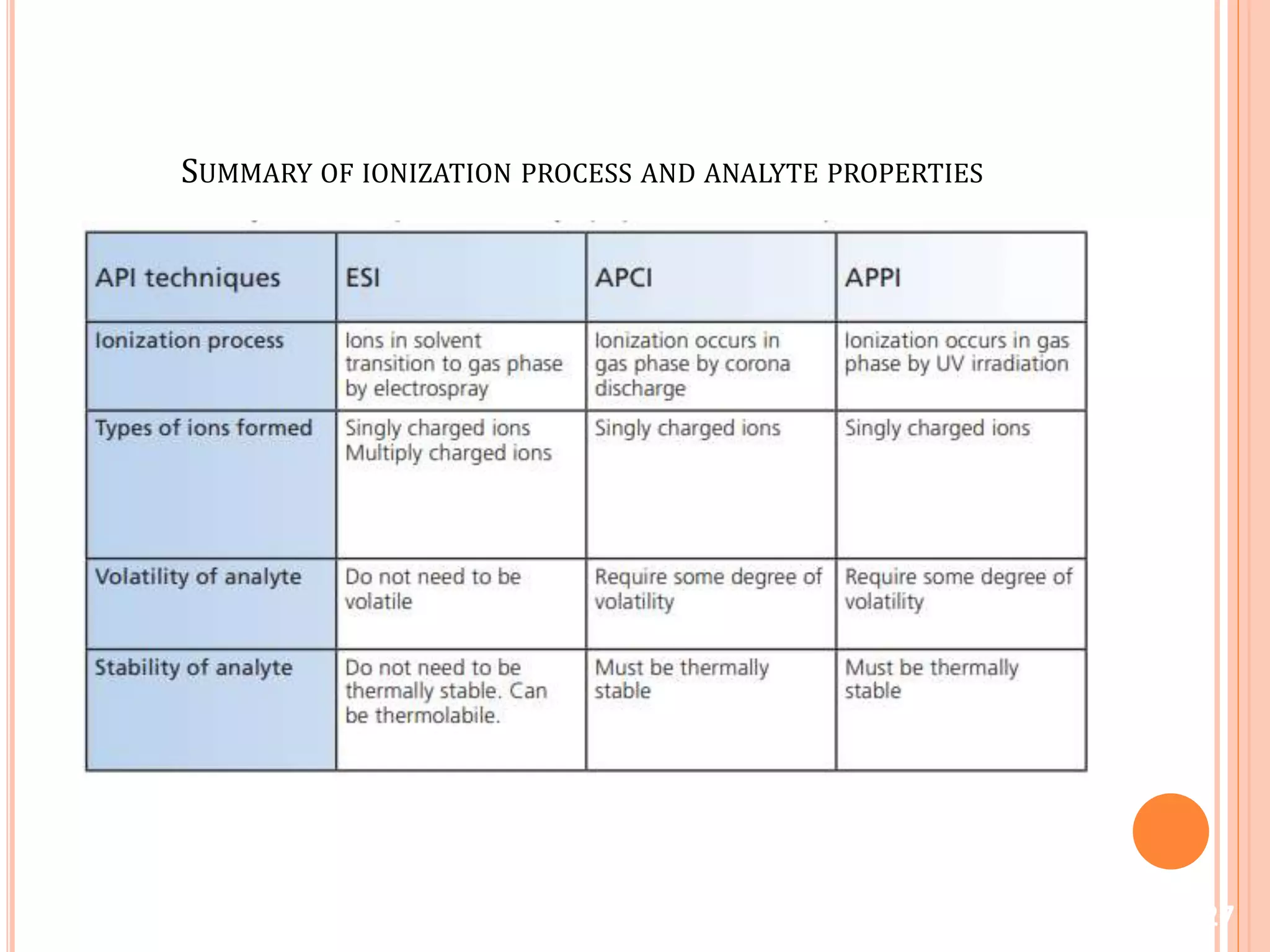
This document provides an overview of LC-MS instrumentation and methodology. It discusses the basic working principles of mass spectrometry and how LC-MS adds additional dimensions of molecular weight and fragmentation information compared to LC alone. The key components of an LC-MS system including the ionization source, mass analyzer, and detector are described. Different scan modes like SIM, precursor ion scan, product ion scan and MRM are also covered. The document provides guidance on setting up an LC-MS method including considerations for column selection, mobile phase composition, sample solvents, buffers, and optimizing for positive or negative ionization of the target analyte.