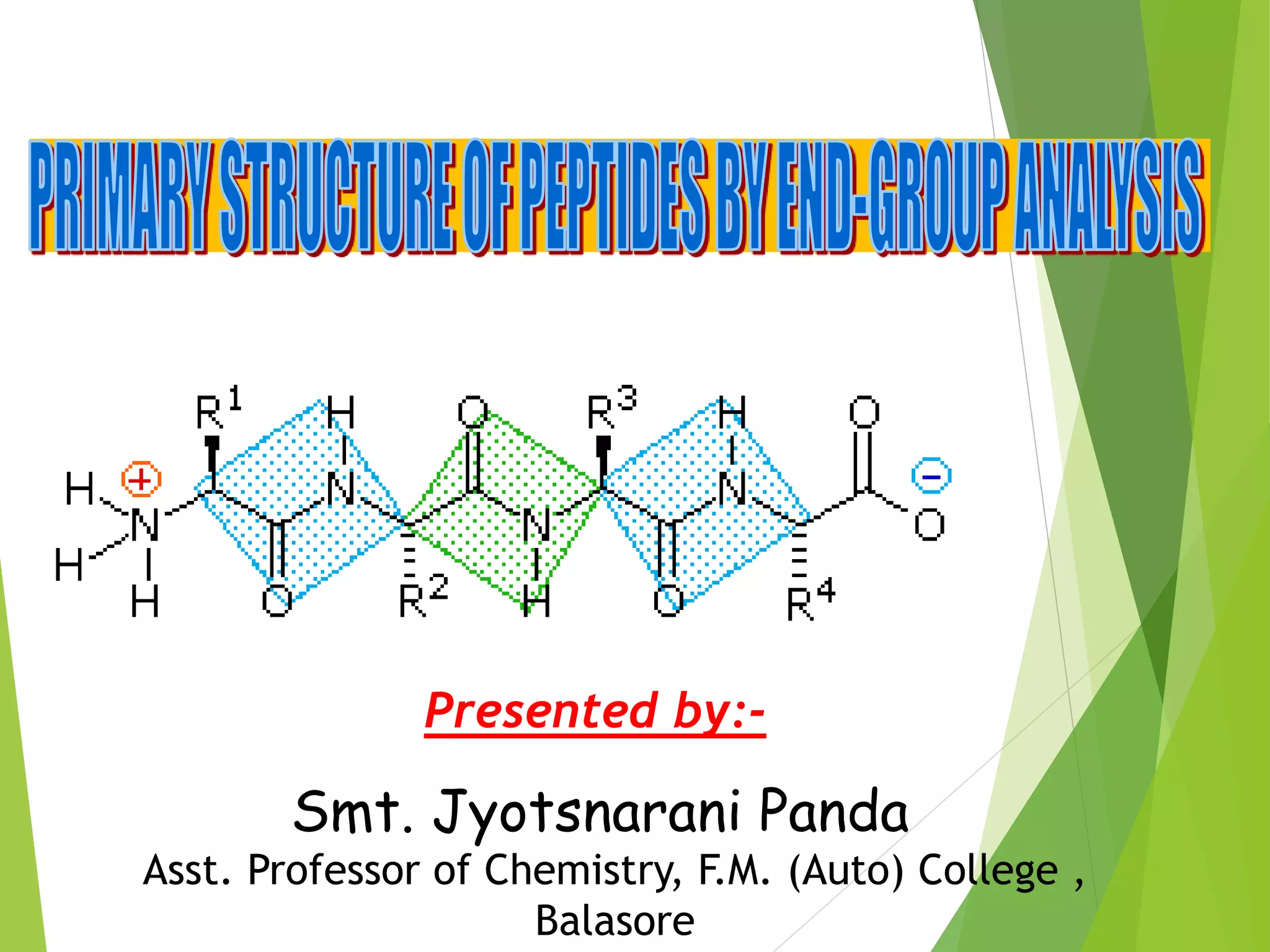
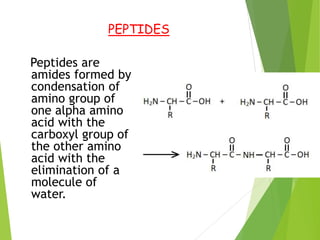
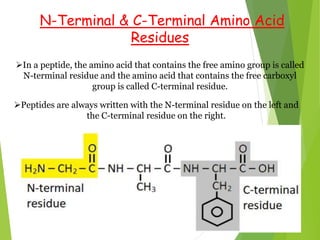
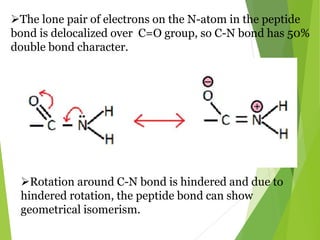
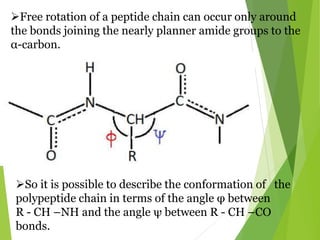
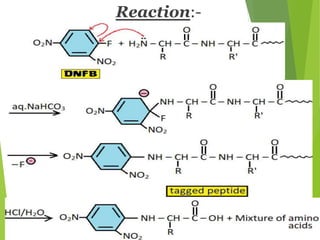
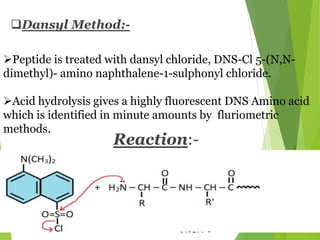
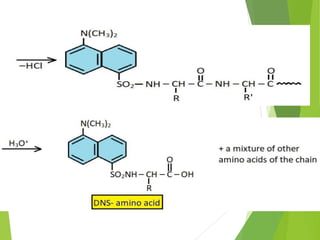
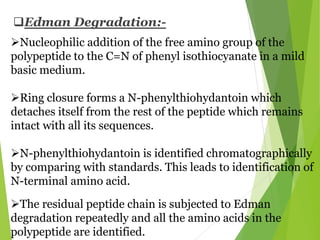
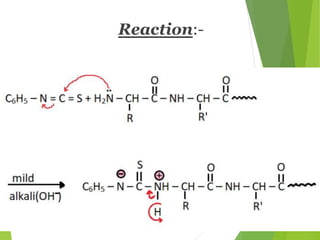
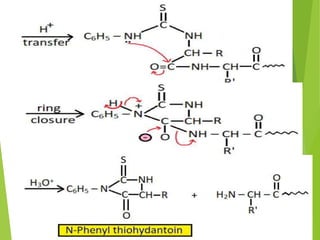
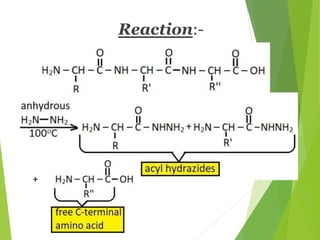
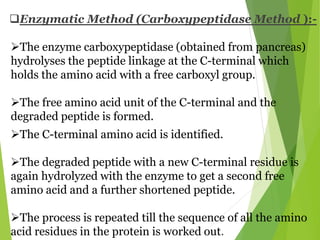
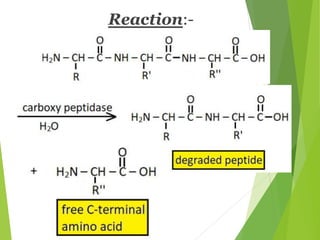
Peptides are formed by the condensation of amino acids, with a specific structure that includes an N-terminal and C-terminal residue. The primary structure refers to the exact sequence of amino acids in a polypeptide chain, which can be determined through methods such as Sanger's or Edman degradation for N-terminal analysis and enzymatic methods for C-terminal analysis. Various chemical reactions and chromatography techniques are employed to identify and analyze amino acids in the peptide sequence.