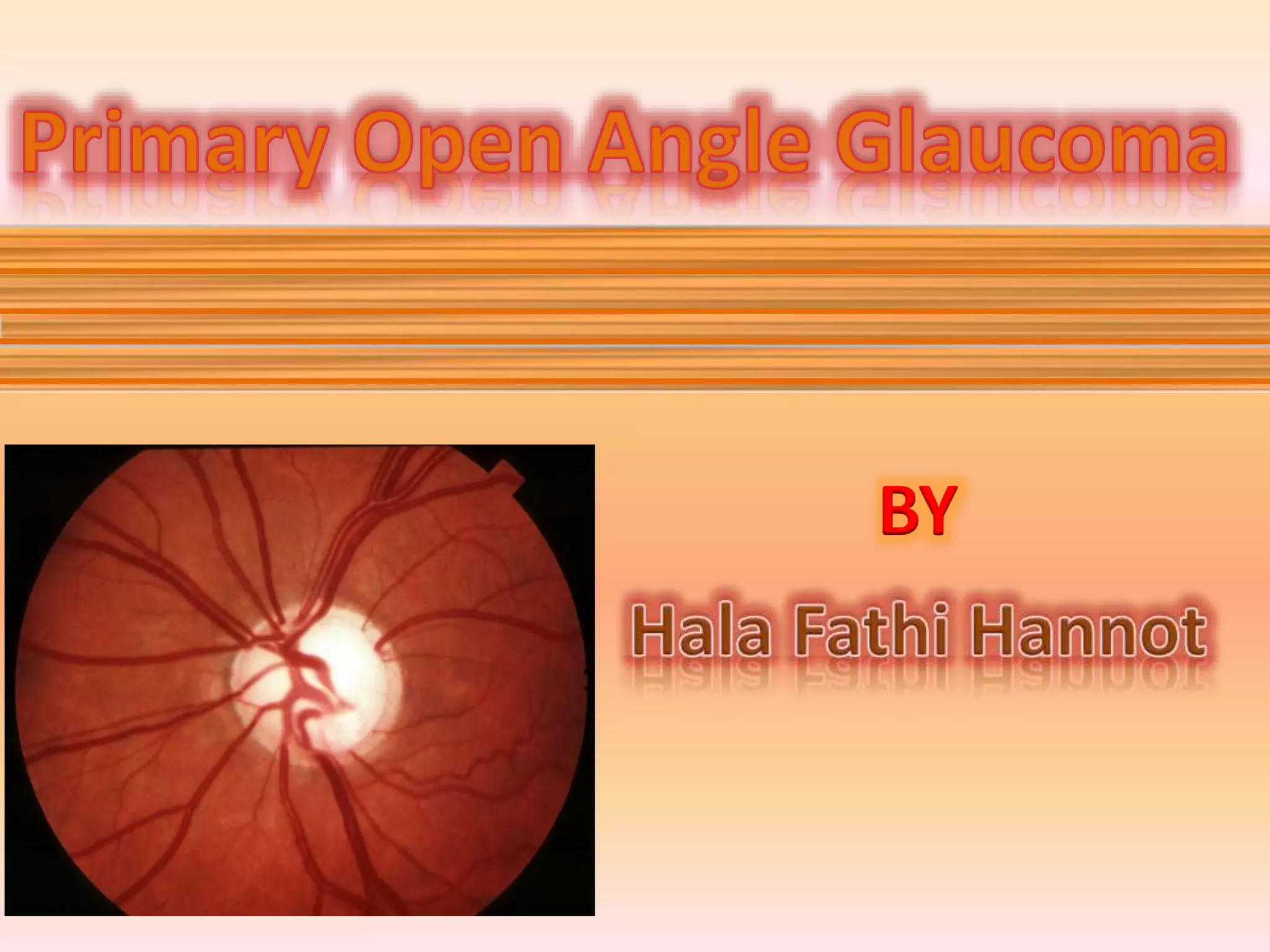
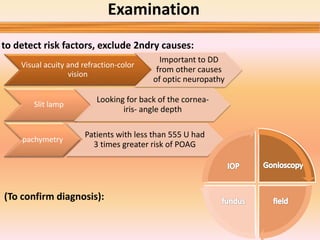
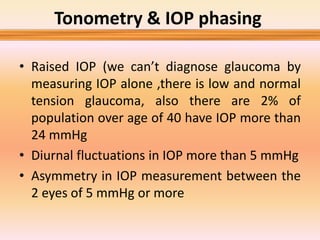
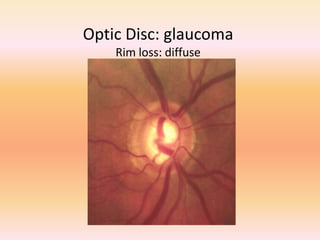
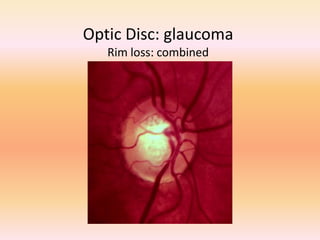
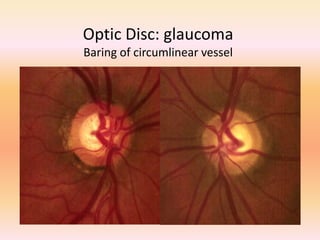
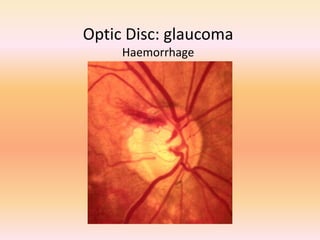
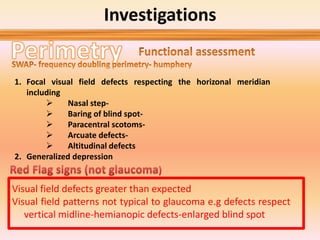
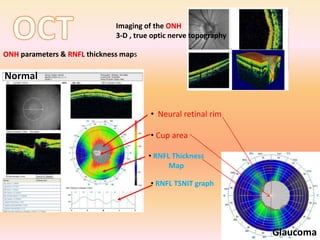
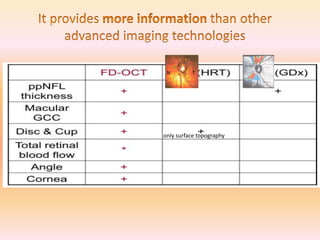
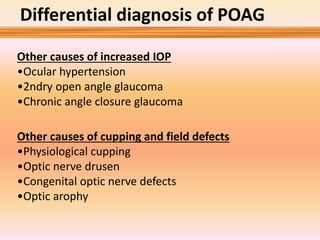
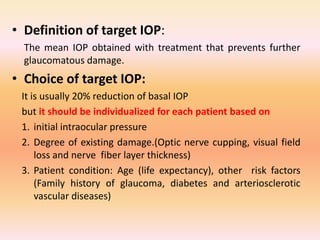
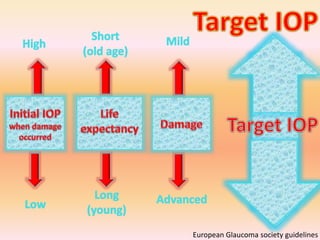
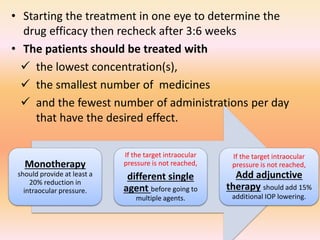
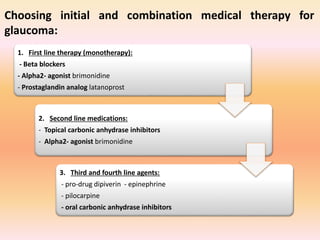
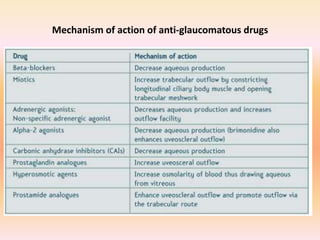
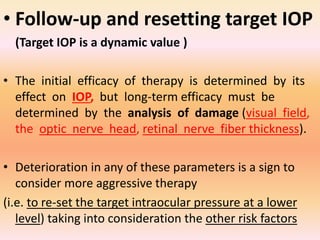
Primary open angle glaucoma is the most common type of glaucoma. It is diagnosed based on raised intraocular pressure, optic nerve damage, and visual field loss. Risk factors include increasing age, family history, and being black. Treatment aims to lower intraocular pressure through medications, laser treatment, or surgery to prevent further optic nerve damage and vision loss. The target intraocular pressure is individualized based on a patient's baseline pressure and degree of existing damage. Ongoing monitoring of the optic nerve and visual fields is needed to ensure the target pressure is maintained and adjusted if needed to stop disease progression.