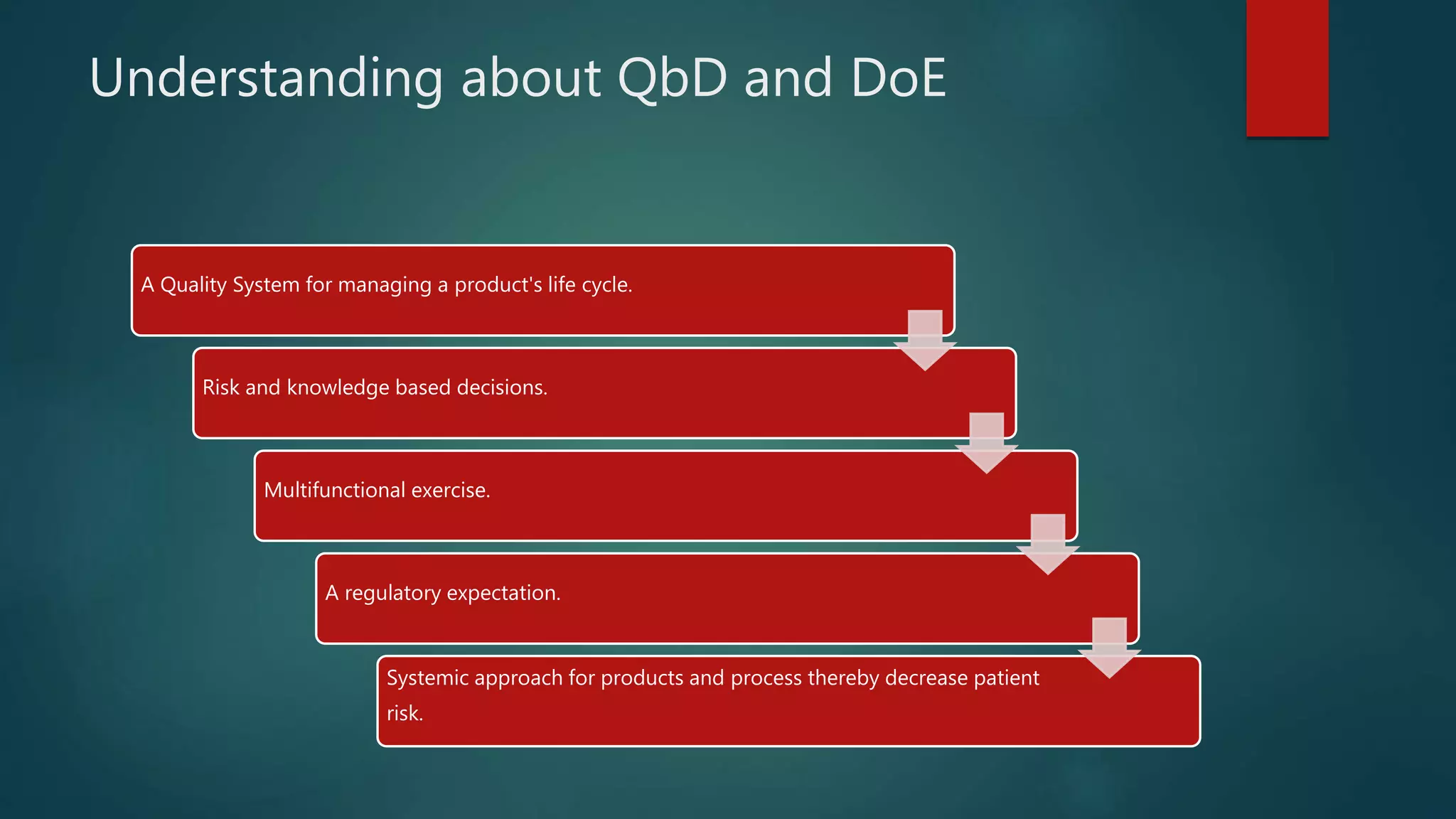
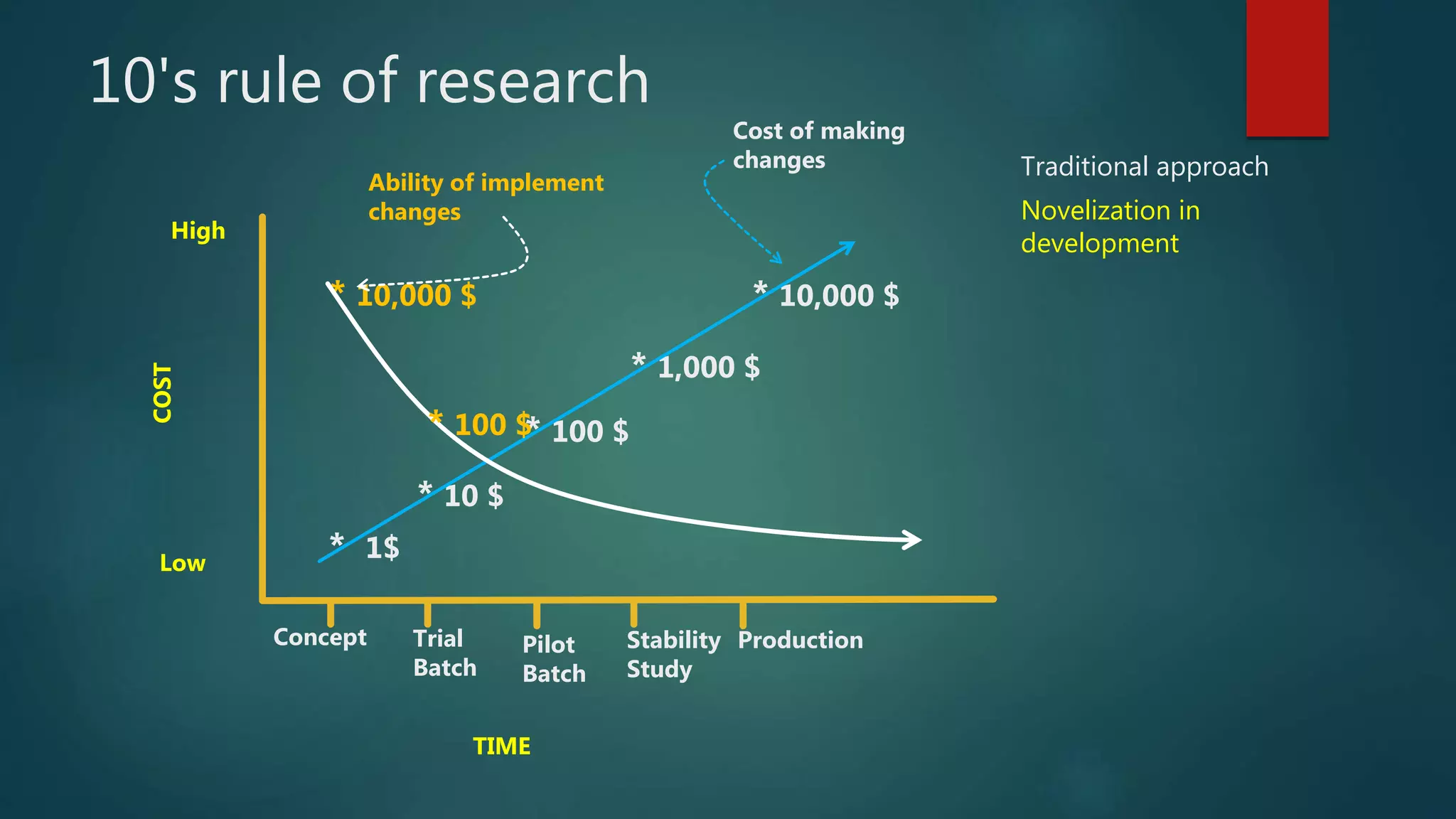
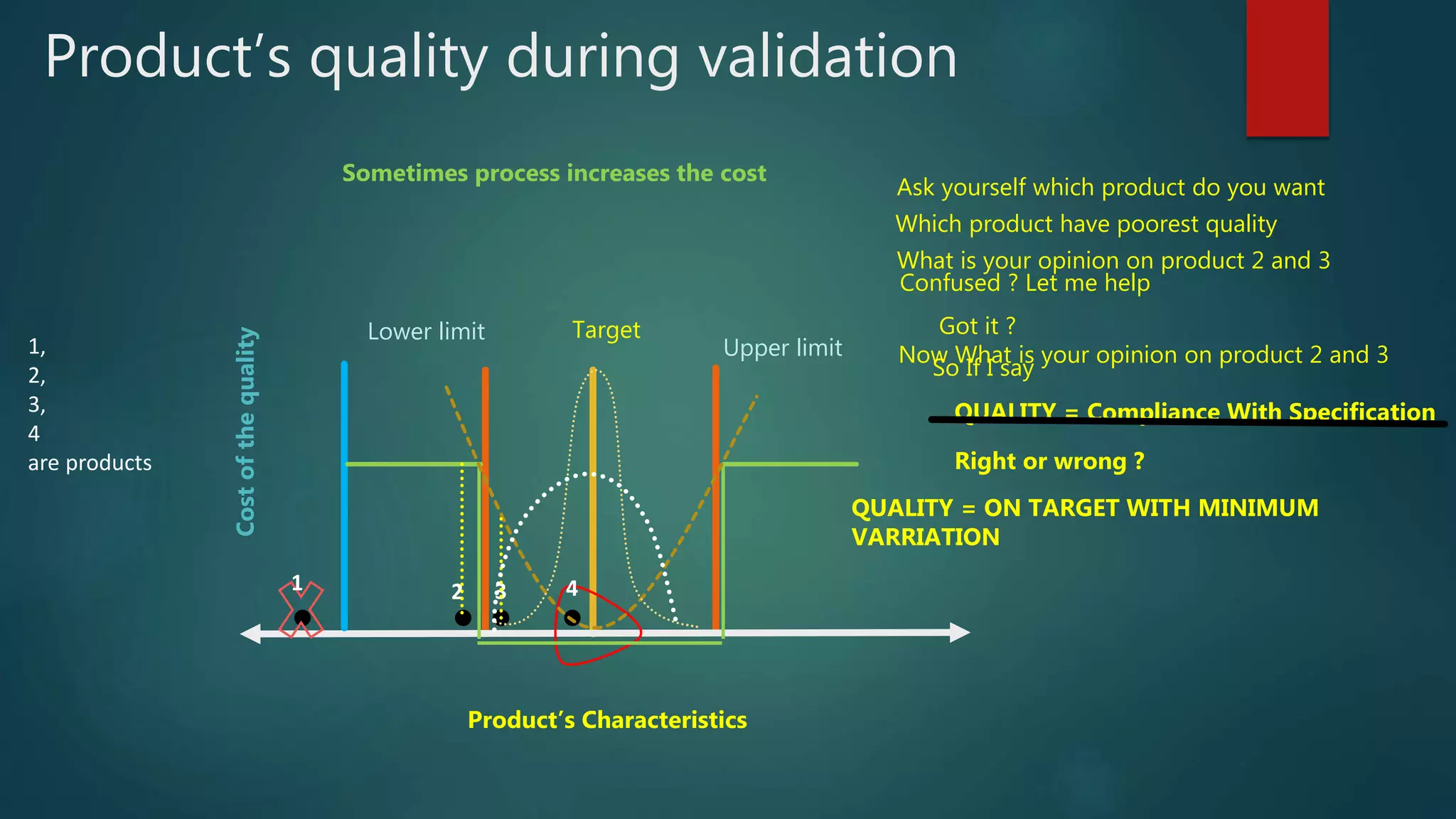
This document discusses Quality by Design (QbD) and Design of Experiments (DoE) principles for drug product development. It aims to develop formulations that reliably deliver the desired pharmacological effects without increasing side effects or losing stability or performance over time. QbD uses a risk-based and knowledge-driven approach to understand critical quality attributes and process parameters that affect product quality. DoE is used to characterize processes and identify sources of variability to ensure consistent manufacturing. The document outlines key ICH guidelines for QbD and DoE and stresses that the goal is achieving a predictable therapeutic response through reproducible, large-scale manufacturing.