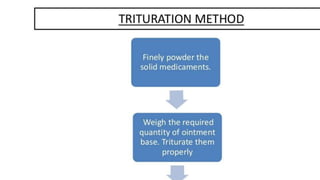
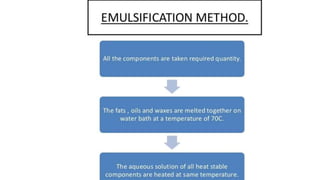
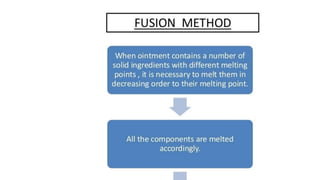
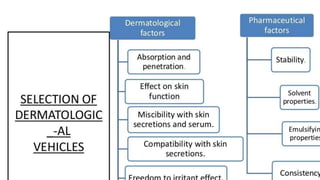
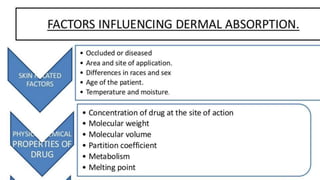
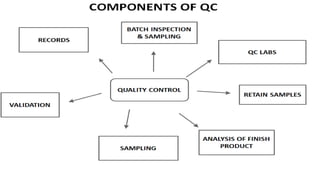
The document discusses the preparation, evaluation, and packaging of ointments, focusing on various tests for assessing absorption, non-irritancy, penetration rate, drug release, and viscosity. It outlines methods for testing microbial content and preservative efficacy, emphasizing the importance of quality assurance in pharmaceutical manufacturing. Additionally, it highlights the uses of a specific product, B Salic cream, containing salicylic acid and benzoic acid for treating skin conditions.