Embed presentation
Download as PDF, PPTX

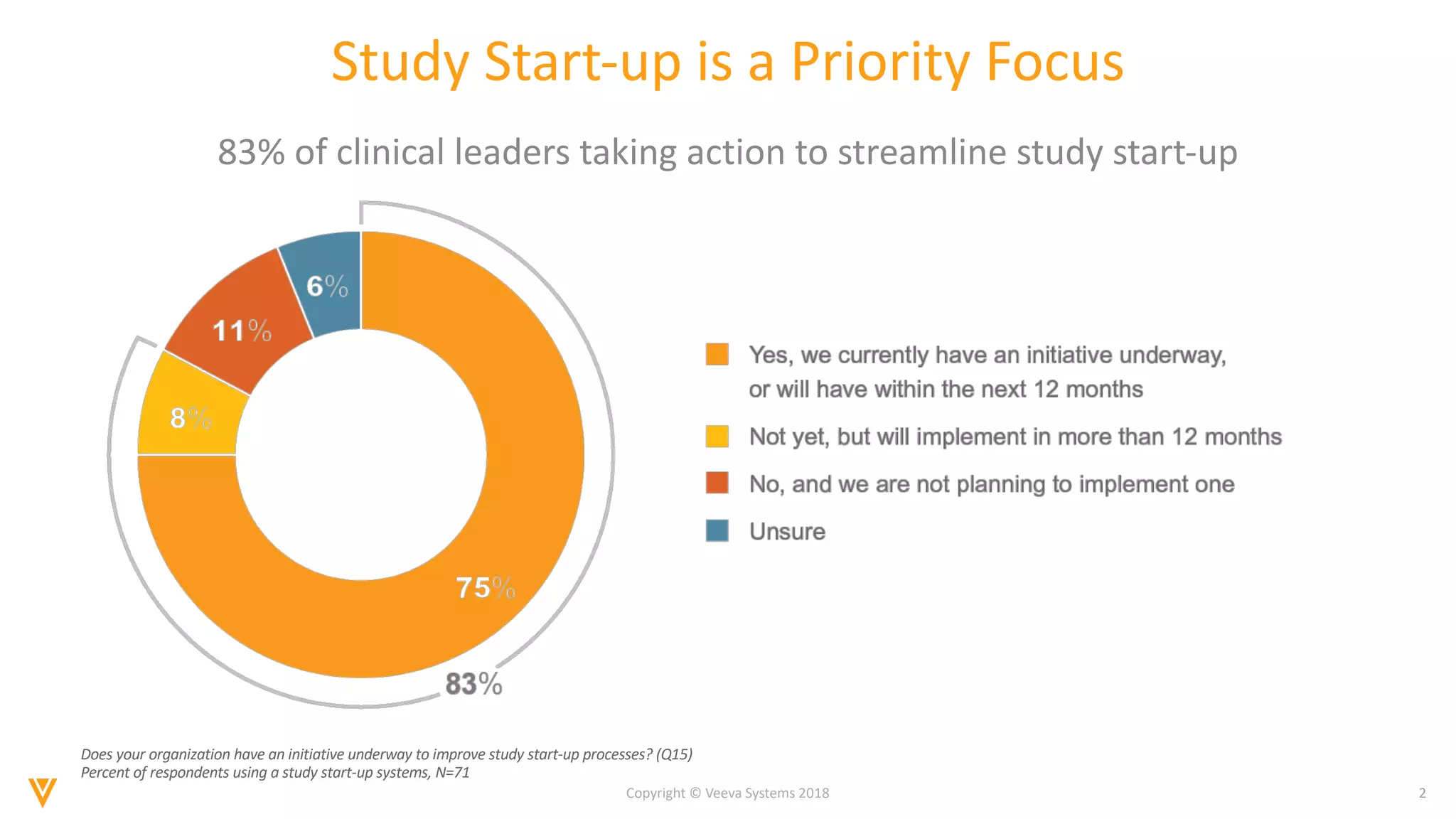
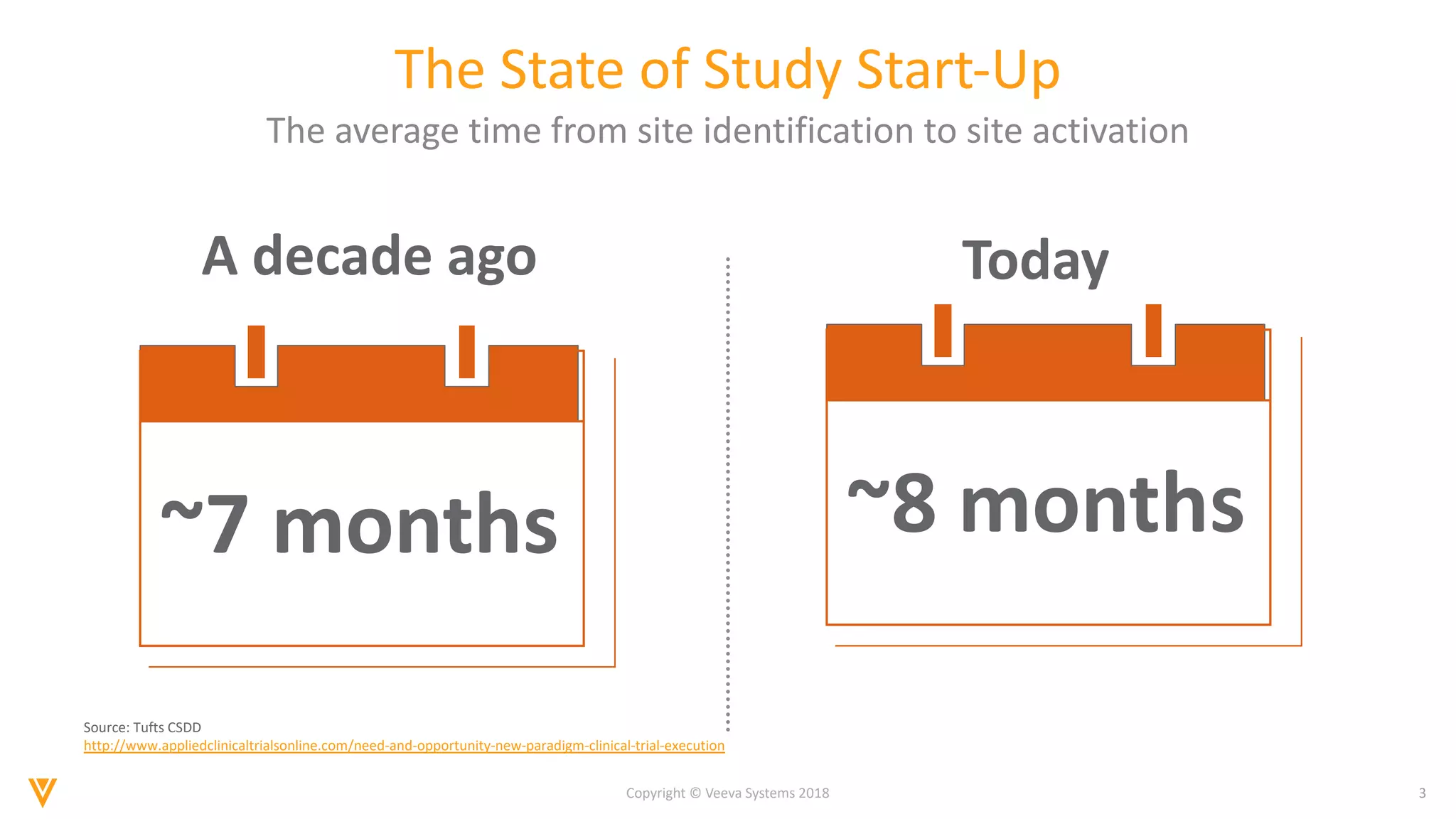
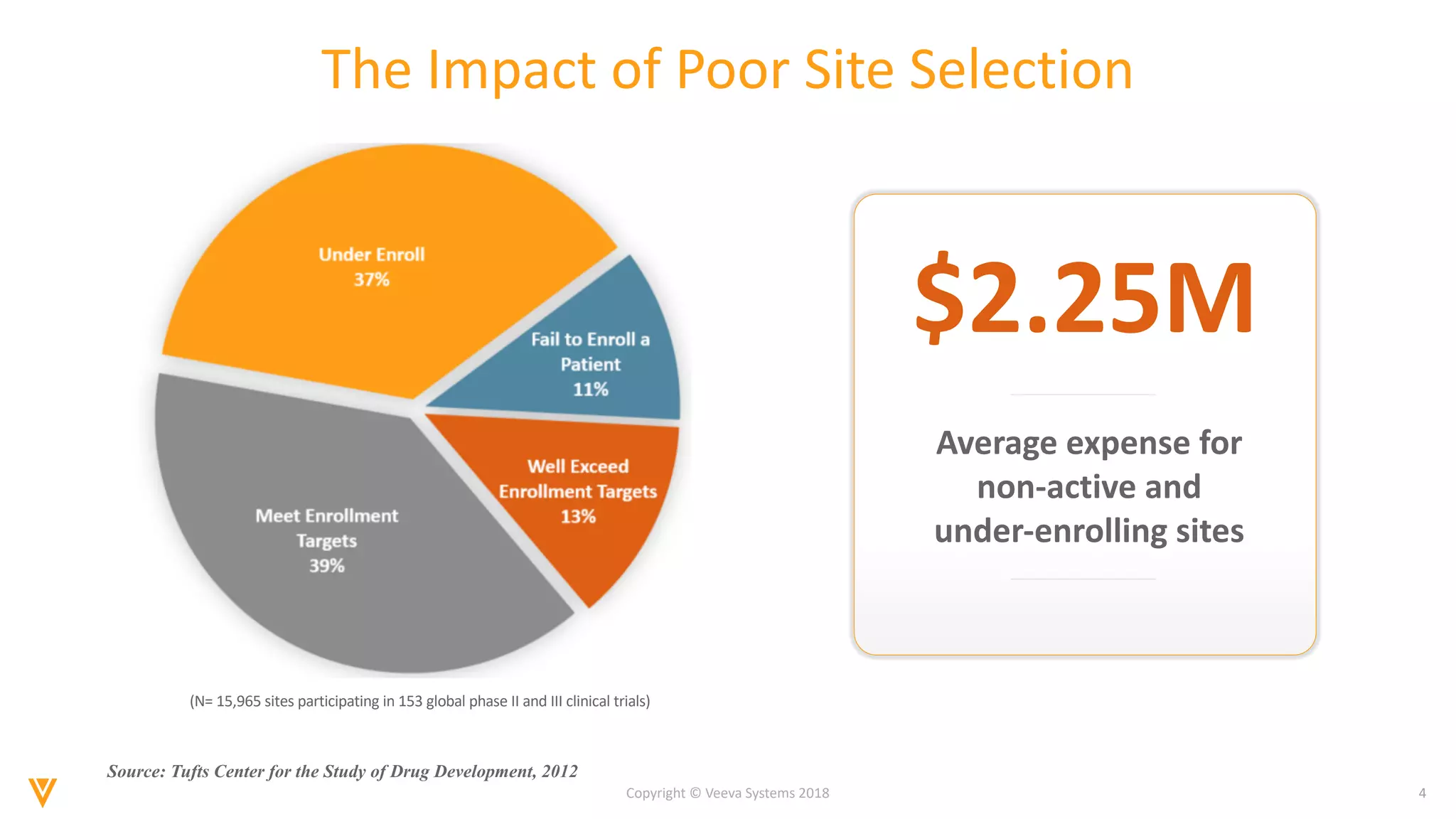
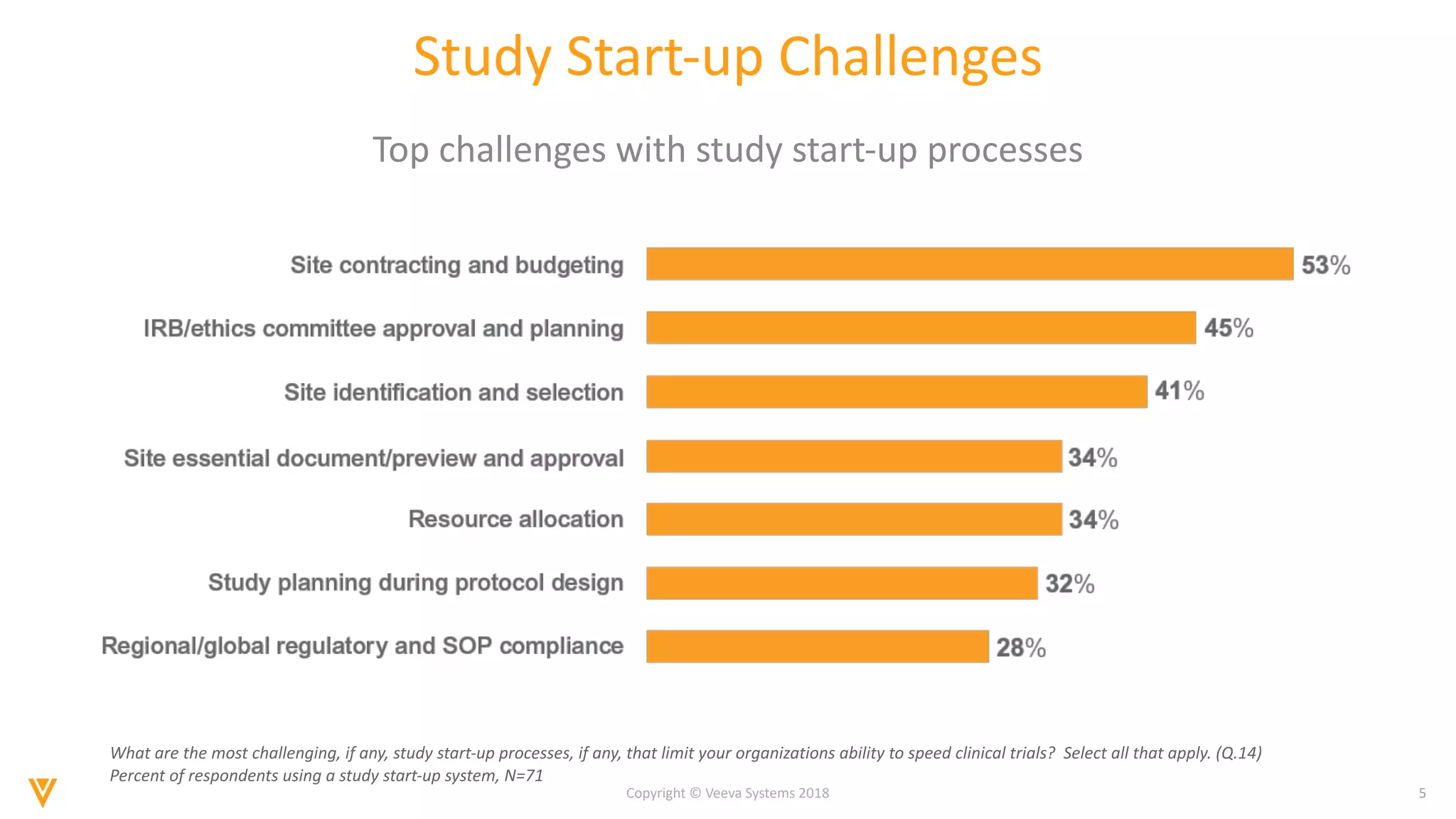
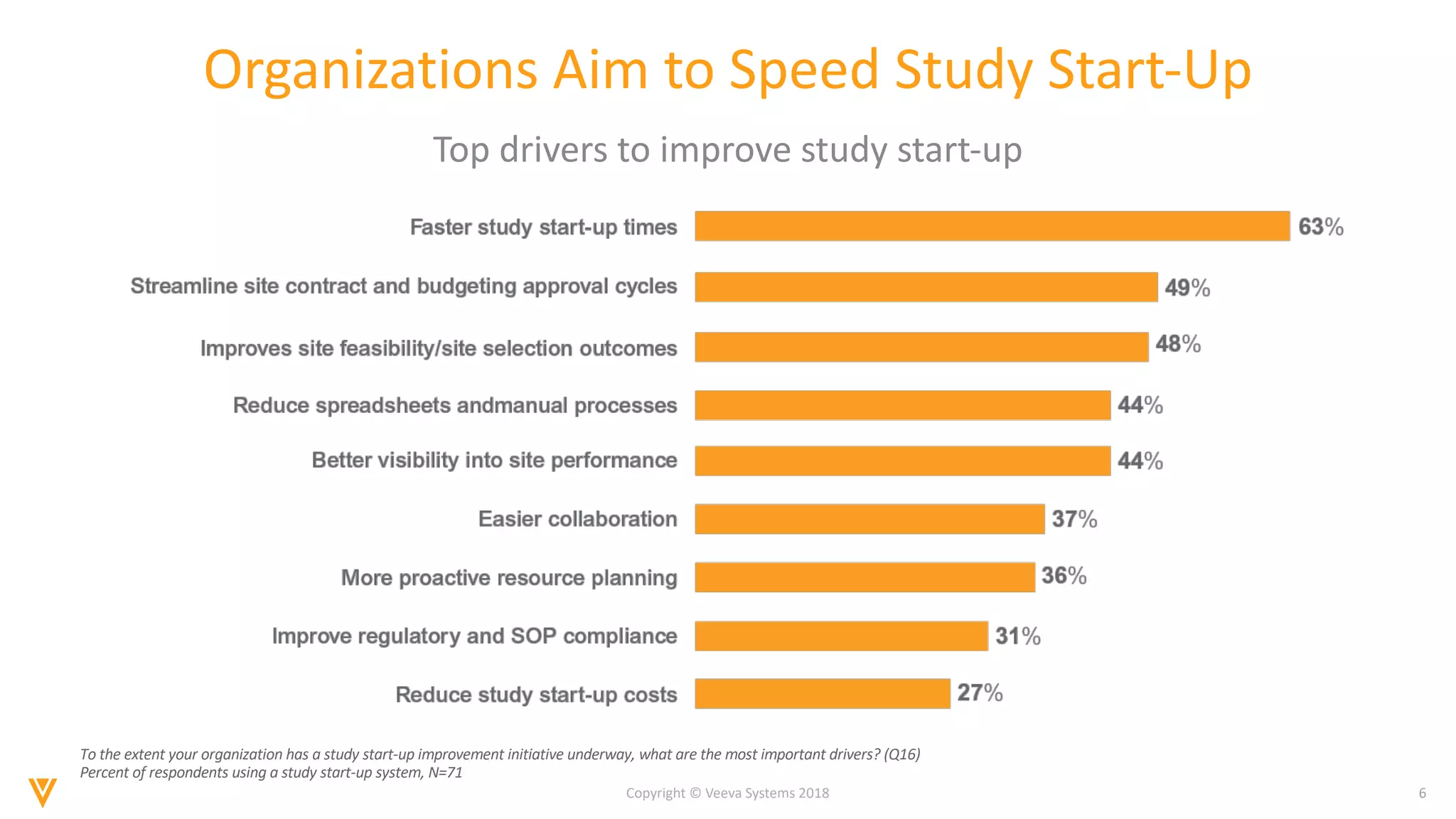


The document discusses findings from a 2018 survey on clinical operations. It shows that 83% of clinical leaders are taking action to streamline study start-up processes, which currently take around 8 months on average. Poor site selection can cost $2.25 million per non-active or under-enrolling site. The top challenges with study start-up processes are site activation and management of regulatory documents. Organizations aim to speed up study start-up primarily to reduce trial costs and timelines.






