The Rise of eCTD: A Visual Explainer
•
0 likes•465 views
The ability to meet standards and regulatory approval is nowhere more important than in the pharmaceutical industry. All companies have responsibilities when it comes to submitting information to authorities in both a precise and proper manner as, otherwise, their latest developments and innovations could be held back by dreaded red tape.
Report
Share
Report
Share
Download to read offline
Recommended
Recommended
CDSCO- CENTRAL DRUG STANDARD CONTROL ORGANISATION

CDSCO- CENTRAL DRUG STANDARD CONTROL ORGANISATIONR.C patel institute of pharmacutical education and research, shirpur
More Related Content
What's hot
CDSCO- CENTRAL DRUG STANDARD CONTROL ORGANISATION

CDSCO- CENTRAL DRUG STANDARD CONTROL ORGANISATIONR.C patel institute of pharmacutical education and research, shirpur
What's hot (20)
Pharma Uptoday Monthly Magazine - Volume 11, Issue Feb 2015

Pharma Uptoday Monthly Magazine - Volume 11, Issue Feb 2015
Pharma Uptoday Monthly Magazine volume 4 issue jul 2014

Pharma Uptoday Monthly Magazine volume 4 issue jul 2014
Formal Meetings between the FDA and Sponsors or Applicants

Formal Meetings between the FDA and Sponsors or Applicants
Czech Republic Patient Monitoring Market Outlook to 2018 - Fetal Monitors, Mu...

Czech Republic Patient Monitoring Market Outlook to 2018 - Fetal Monitors, Mu...
Pharma Uptoday Monthly Magazine Volume 5, Issue Aug 2014

Pharma Uptoday Monthly Magazine Volume 5, Issue Aug 2014
Pharma Uptoday Monthly Magazine - Volume 10 issue Jan 2015

Pharma Uptoday Monthly Magazine - Volume 10 issue Jan 2015
Poland Patient Monitoring Investment Opportunities, Analysis and Forecasts to...

Poland Patient Monitoring Investment Opportunities, Analysis and Forecasts to...
Greece Anesthesia and Respiratory Devices Investment Opportunities, Analysis ...

Greece Anesthesia and Respiratory Devices Investment Opportunities, Analysis ...
Comparison of Clinical Trial Application requirement of India, USA and Europe.

Comparison of Clinical Trial Application requirement of India, USA and Europe.
Pharma Uptoday Monthly Magazine - Volume 19; issue: Oct 2015

Pharma Uptoday Monthly Magazine - Volume 19; issue: Oct 2015
Pharma Uptoday Monthly Magazine - Volume 18; Issue: Sep 2015

Pharma Uptoday Monthly Magazine - Volume 18; Issue: Sep 2015
Pharma Uptoday Monthly Magazine Volume 22; Issue Jan 2016

Pharma Uptoday Monthly Magazine Volume 22; Issue Jan 2016
Pharma Uptoday Monthly Magazine Volume 8 issue Nov 2014

Pharma Uptoday Monthly Magazine Volume 8 issue Nov 2014
Similar to The Rise of eCTD: A Visual Explainer
Similar to The Rise of eCTD: A Visual Explainer (20)
REGISTRATION OF INDIAN DRUG PRODUCT IN OVERSEAS MARKET.pptx

REGISTRATION OF INDIAN DRUG PRODUCT IN OVERSEAS MARKET.pptx
Premarket Notification 510(k) for Biologics [Autosaved].pptx![Premarket Notification 510(k) for Biologics [Autosaved].pptx](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Premarket Notification 510(k) for Biologics [Autosaved].pptx](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
Premarket Notification 510(k) for Biologics [Autosaved].pptx
Sanitizer & Disinfectants During COVID 19 – A Brief Study

Sanitizer & Disinfectants During COVID 19 – A Brief Study
Canadian Medical Devices Conformity Assessment System(CMDCAS) - Training mate...

Canadian Medical Devices Conformity Assessment System(CMDCAS) - Training mate...
CE Marking , FDA Approval and Associated Regulations for Wellness

CE Marking , FDA Approval and Associated Regulations for Wellness
Portugal Cardiovascular Devices Investment Opportunities, Analysis and Foreca...

Portugal Cardiovascular Devices Investment Opportunities, Analysis and Foreca...
More from Montrium
More from Montrium (20)
Monitoring Beyond COVID-19: Setting Yourself Up for the New-Normal

Monitoring Beyond COVID-19: Setting Yourself Up for the New-Normal
Best Practices for Implementing Robust Governance Processes in Office 365

Best Practices for Implementing Robust Governance Processes in Office 365
Strategies to Facilitate GxP Processes Deployment in Office 365

Strategies to Facilitate GxP Processes Deployment in Office 365
How to Get Started with GxP Processes in Office 365 - The Discovery Phase

How to Get Started with GxP Processes in Office 365 - The Discovery Phase
How to prepare for an audit and maintain oversight within your e qms

How to prepare for an audit and maintain oversight within your e qms
Transforming eTMF Management: Moving to a Data-Driven Approach

Transforming eTMF Management: Moving to a Data-Driven Approach
Best practices for preparing for and surviving inspections

Best practices for preparing for and surviving inspections
Best practices for preparing for and surviving inspections

Best practices for preparing for and surviving inspections
Implementing Metrics & Completeness Reporting in TMF Management

Implementing Metrics & Completeness Reporting in TMF Management
Empowering Active TMF Management With an eTMF System

Empowering Active TMF Management With an eTMF System
Automation of document management paul fenton webinar

Automation of document management paul fenton webinar
Practical Steps to Selecting and Implementing an eTMF

Practical Steps to Selecting and Implementing an eTMF
TMF Fundamentals - An Introduction to Better Trial Master File Management - M...

TMF Fundamentals - An Introduction to Better Trial Master File Management - M...
Strategies for Conducting GxP Vendor Assessment of Cloud Service Providers - ...

Strategies for Conducting GxP Vendor Assessment of Cloud Service Providers - ...
Recently uploaded
Recently uploaded (20)
Scanning the Internet for External Cloud Exposures via SSL Certs

Scanning the Internet for External Cloud Exposures via SSL Certs
Ensuring Technical Readiness For Copilot in Microsoft 365

Ensuring Technical Readiness For Copilot in Microsoft 365
"Subclassing and Composition – A Pythonic Tour of Trade-Offs", Hynek Schlawack

"Subclassing and Composition – A Pythonic Tour of Trade-Offs", Hynek Schlawack
Hyperautomation and AI/ML: A Strategy for Digital Transformation Success.pdf

Hyperautomation and AI/ML: A Strategy for Digital Transformation Success.pdf
Take control of your SAP testing with UiPath Test Suite

Take control of your SAP testing with UiPath Test Suite
Designing IA for AI - Information Architecture Conference 2024

Designing IA for AI - Information Architecture Conference 2024
"LLMs for Python Engineers: Advanced Data Analysis and Semantic Kernel",Oleks...

"LLMs for Python Engineers: Advanced Data Analysis and Semantic Kernel",Oleks...
New from BookNet Canada for 2024: BNC CataList - Tech Forum 2024

New from BookNet Canada for 2024: BNC CataList - Tech Forum 2024
WordPress Websites for Engineers: Elevate Your Brand

WordPress Websites for Engineers: Elevate Your Brand
DevoxxFR 2024 Reproducible Builds with Apache Maven

DevoxxFR 2024 Reproducible Builds with Apache Maven
Unraveling Multimodality with Large Language Models.pdf

Unraveling Multimodality with Large Language Models.pdf
DSPy a system for AI to Write Prompts and Do Fine Tuning

DSPy a system for AI to Write Prompts and Do Fine Tuning
TrustArc Webinar - How to Build Consumer Trust Through Data Privacy

TrustArc Webinar - How to Build Consumer Trust Through Data Privacy
Unleash Your Potential - Namagunga Girls Coding Club

Unleash Your Potential - Namagunga Girls Coding Club
Gen AI in Business - Global Trends Report 2024.pdf

Gen AI in Business - Global Trends Report 2024.pdf
The Rise of eCTD: A Visual Explainer
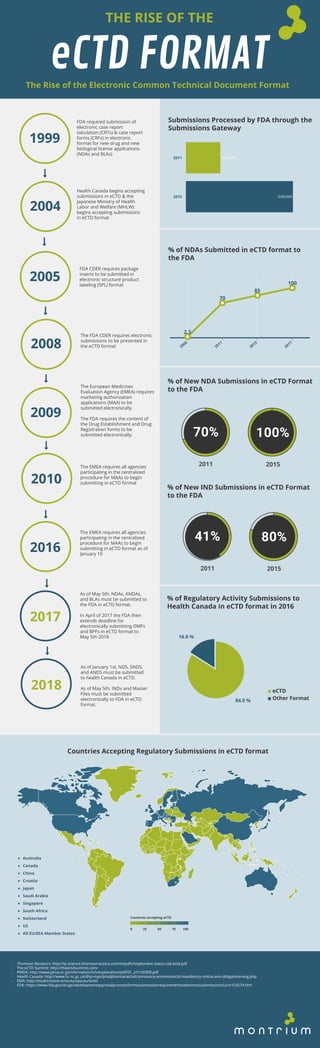
- 1. THE RISE OF THE eCTD FORMATThe Rise of the Electronic Common Technical Document Format 1999 2004 2005 2008 2009 FDA required submission of electronic case report tabulation (CRTs) & case report forms (CRFs) in electronic format for new drug and new biological license applications (NDAs and BLAs) Health Canada begins accepting submissions in eCTD & the Japanese Ministry of Health Labor and Welfare (MHLW) begins accepting submissions in eCTD format FDA CDER requires package inserts to be submitted in electronic structure product labeling (SPL) format The FDA CDER requires electronic submissions to be presented in the eCTD format The European Medicines Evaluation Agency (EMEA) requires marketing authorization applications (MAA) to be submitted electronically. The FDA requires the content of the Drug Establishment and Drug Registration forms to be submitted electronically. 1,000,000 3,100,000 2011 2015 Submissions Processed by FDA through the Submissions Gateway 2.3 70 85 100 2006 2011 2015 2017 % of NDAs Submitted in eCTD format to the FDA % of New NDA Submissions in eCTD Format to the FDA 70% 100% 2011 2015 % of New IND Submissions in eCTD Format to the FDA 41% 80% 2011 2015 2010 The EMEA requires all agencies participating in the centralized procedure for MAAs to begin submitting in eCTD format 2016 The EMEA requires all agencies participating in the centralized procedure for MAAs to begin submitting in eCTD format as of January 10 2017 As of May 5th, NDAs, ANDAs, and BLAs must be submitted to the FDA in eCTD format. In April of 2017 the FDA then extends deadline for electronically submitting DMFs and BPFs in eCTD format to May 5th 2018 2018 As of January 1st, NDS, SNDS, and ANDS must be submitted to health Canada in eCTD. As of May 5th, INDs and Master Files must be submitted electronically to FDA in eCTD format. 84.0 % 16.0 % eCTD Other Format % of Regulatory Activity Submissions to Health Canada in eCTD format in 2016 0 25 50 75 100 Countries accepting eCTD Countries Accepting Regulatory Submissions in eCTD format - Thomson Reuteurs: http://ip-science.thomsonreuters.com/m/pdfs/implement-status-ctd-ectd.pdf - The eCTD Summit: http://theectdsummit.com/ - PMDA: http://www.jpma.or.jp/information/ich/explanation/pdf/01_ich150309.pdf - Health Canada: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/announce-annonce/ectd-mandatory-notice-avis-obligatoire-eng.php - EMA: http://esubmission.ema.europa.eu/ectd/ - FDA: https://www.fda.gov/drugs/developmentapprovalprocess/formssubmissionrequirements/electronicsubmissions/ucm153574.htm Australia Canada China Croatia Japan Saudi Arabia Singapore South Africa Switzerland US All EU/EEA Member States
