Comparison Between Tablets Granulation Methods
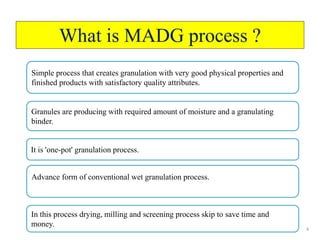
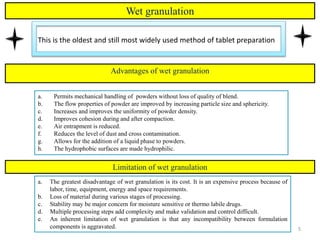
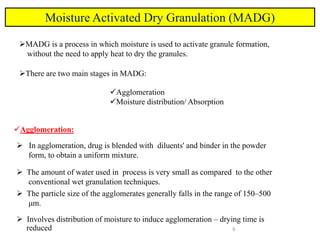
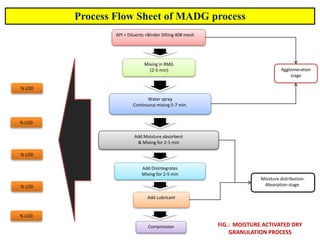
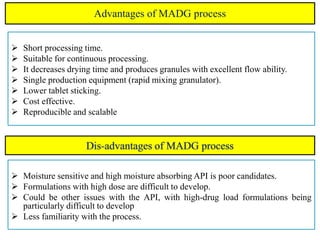
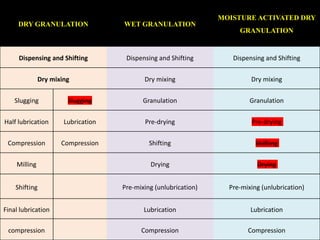
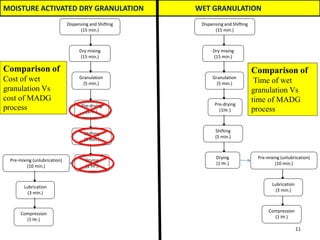
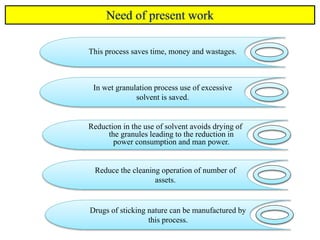
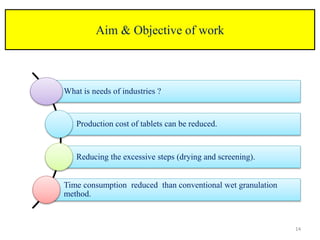
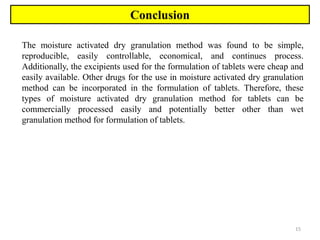
The document compares different tablet granulation methods including wet granulation, dry granulation, and moisture activated dry granulation (MADG). MADG is described as a simple, one-pot process that creates granules using a small amount of water without the need for drying. It saves time and money compared to wet granulation by skipping drying, milling, and screening steps. MADG results in granules with good physical properties and flowability at a lower cost than conventional wet granulation.