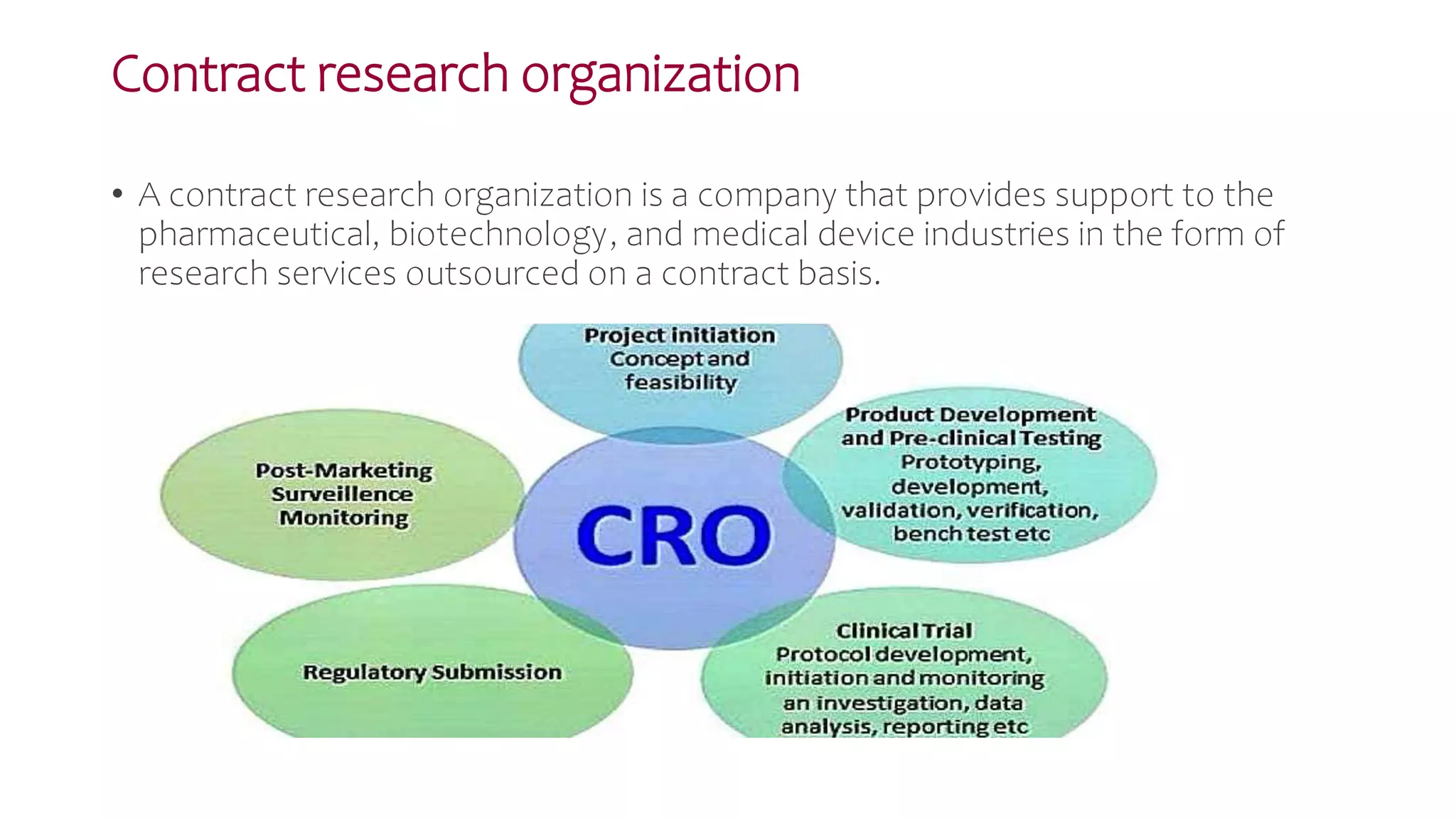
The document discusses the roles and responsibilities of clinical trial sponsors and contract research organizations (CROs). It outlines that sponsors are responsible for initiating, managing, and financing clinical trials in accordance with good clinical practice guidelines. They must select investigators, monitor trials, ensure regulatory approval is obtained, and address safety issues. CROs assist sponsors by providing outsourced clinical research services like monitoring, data management, and regulatory support. Sponsors and CROs have legal contracts defining their responsibilities for conducting research properly and protecting participant safety.