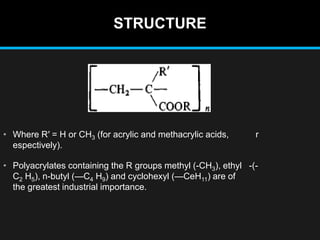
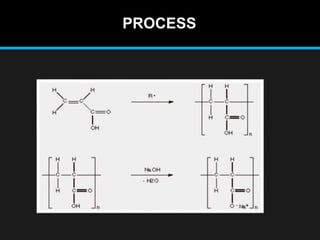
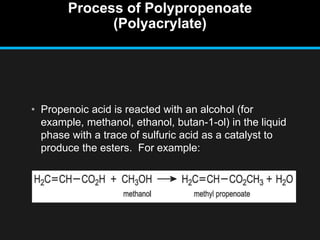
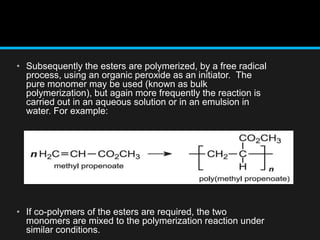
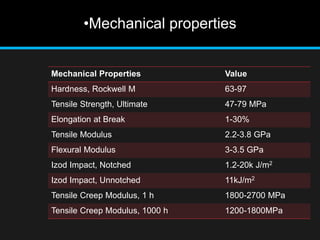
Polyacrylate, known as prop-2-enoic acid, is a versatile acrylic polymer prized for its transparency, elasticity, and water absorption capabilities. Historically evolved from fiber-based materials, it now serves numerous applications in agriculture, hygiene products, and other industries, notably enhancing seed germination and absorbing excess moisture. While beneficial for its absorbent properties and cost-effectiveness, polyacrylate has limitations in durability against certain chemicals and conditions.