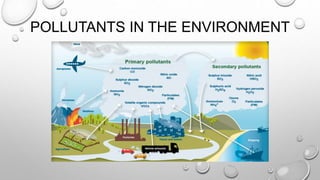
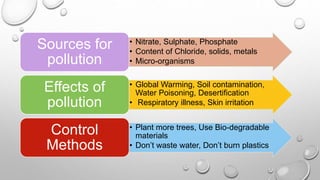
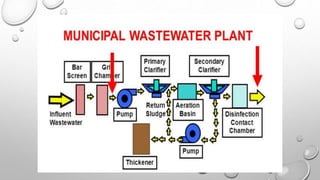
This document discusses pollution monitoring and control methods. It introduces different types of pollutants like nitrate, sulfate, and phosphate that come from sources such as fertilizers, industrial effluents, and sewage. These pollutants can cause eutrophication of water bodies and acid rain. The document then explains methods used to monitor waste water treatment like chemical oxygen demand, biochemical oxygen demand, and total organic carbon. It concludes by emphasizing the importance of preventing pollution to protect the environment and emphasizes reducing, reusing, and recycling to control pollution.