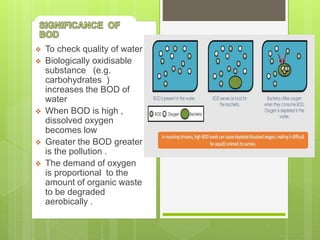
BOD measures the amount of dissolved oxygen required by aerobic organisms to break down organic matter over 5 days, while COD measures the oxygen required to chemically oxidize organic compounds using a strong chemical oxidant. BOD uses a biological oxidation process that is slower but measures naturally degradable organics, while COD uses a chemical oxidation process that is faster but measures all organics including those not degraded biologically. COD values are typically higher than BOD and are used to measure pollution from industrial sources.