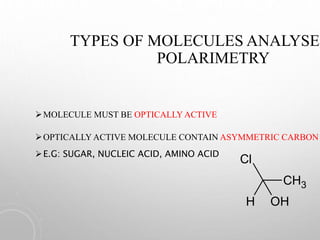
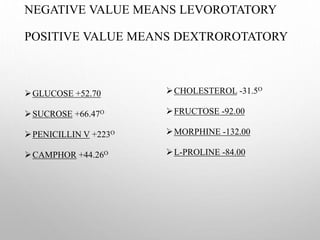
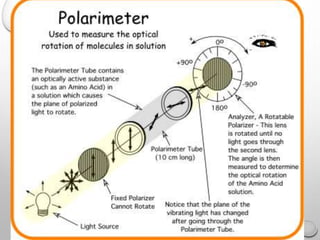
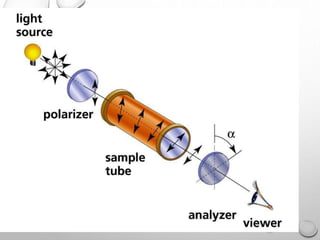
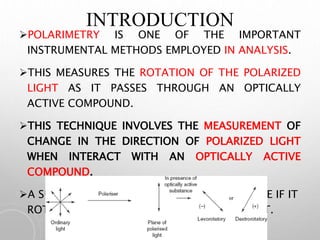
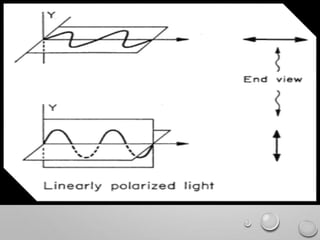
Polarimetry is a technique that measures the rotation of polarized light after it passes through an optically active compound. It involves using a polarimeter instrument containing a light source, polarizer, sample tube containing the compound, and analyzer. The technique is used to quantify concentration and qualitatively distinguish between isomers by measuring their specific rotation values. It has applications in pharmaceutical, chemical, food, and other industries for quality control and research.




![SPECIFIC ROTATION
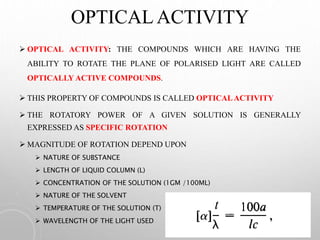
THE ROTATORY POWER OF A GIVEN SOLUTION IS
GENERALLY EXPRESSED AS SPECIFIC ROTATION
IT IS THE NUMBER OF DEGRESS OF ROTATION OF PLANE
POLARIZED LIGHT PRODUCED BY ONE GRAM OF THE
SUBSTANCE PER ML.
MEASUREMENTS IS CARRIED OUT AT TEMP USING
SODIUM LIGHT.
[α] = specific rotation, T = temperature,
λ = wavelength, θ= optical rotation,
c = concentration in g/100ml, l = optical path length in dm.](https://image.slidesharecdn.com/polarimeter-1-201025091025/85/Polarimeter-8-320.jpg)