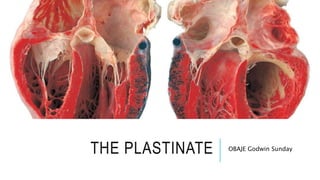
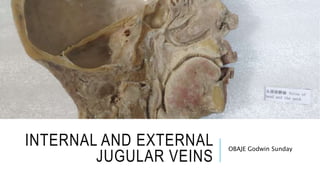
Plastination is a method for long-term preservation of biological tissues, developed by Dr. Gunther von Hagens in 1978, which replaces water and fat with polymers to create durable and odorless specimens called plastinates. The process includes four main steps: fixation, dehydration, forced impregnation in a vacuum, and hardening, requiring specific equipment and chemicals, and is aimed at enhancing educational tools in medical studies. Despite its advantages over traditional formalin preservation, plastination is costly, time-consuming, and requires skilled handling due to the safety risks associated with the chemicals used.



















![FORCED IMPREGNATION IN
VACCUM CONT’D
Structure of hydroxyl- terminated polydimethyl siloxane.
[Si - O] represents a basic silicone molecule.](https://image.slidesharecdn.com/plastination1-191030083458/85/Plastination-by-OBAJE-Godwin-Sunday-24-320.jpg)




