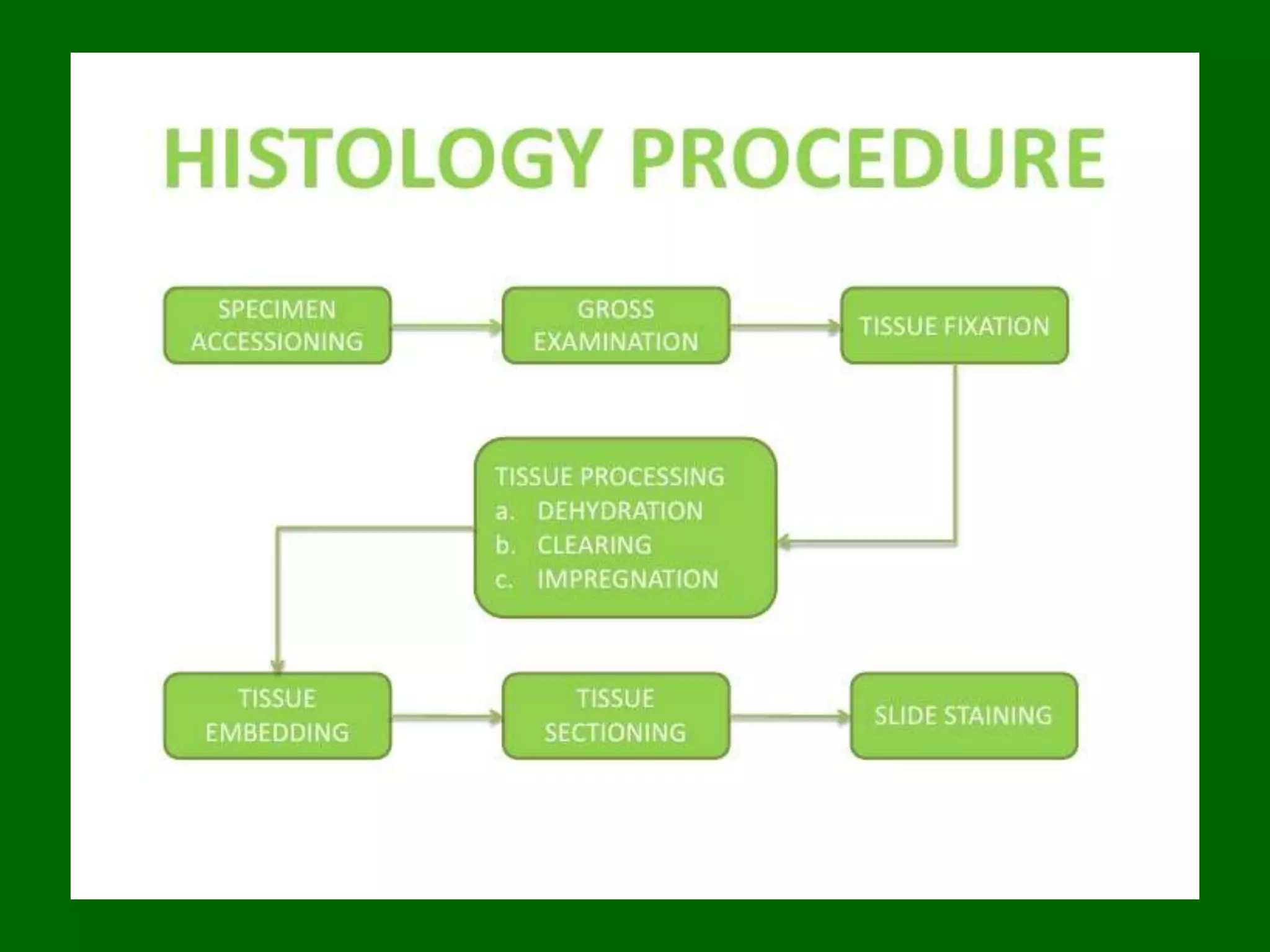
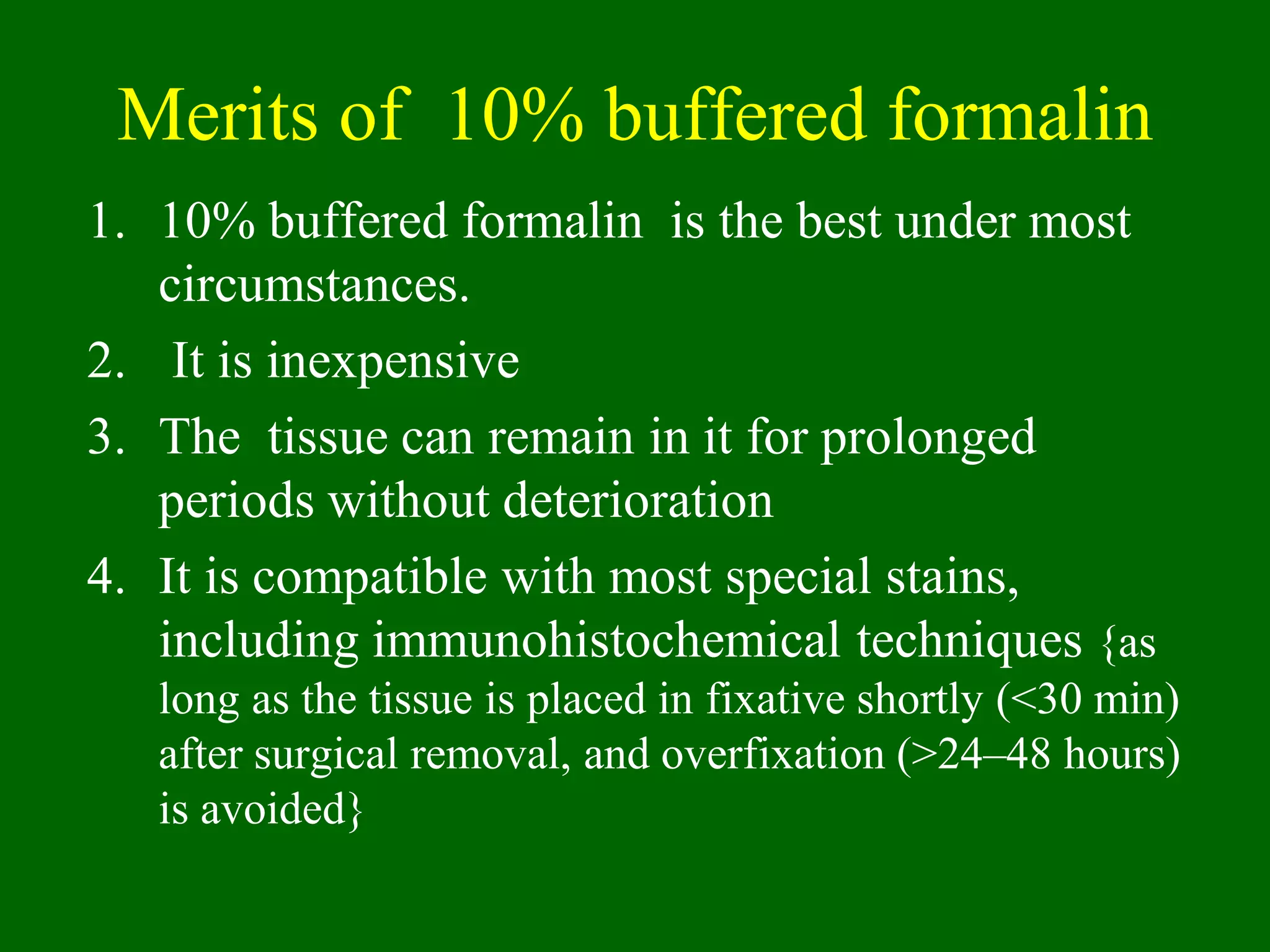
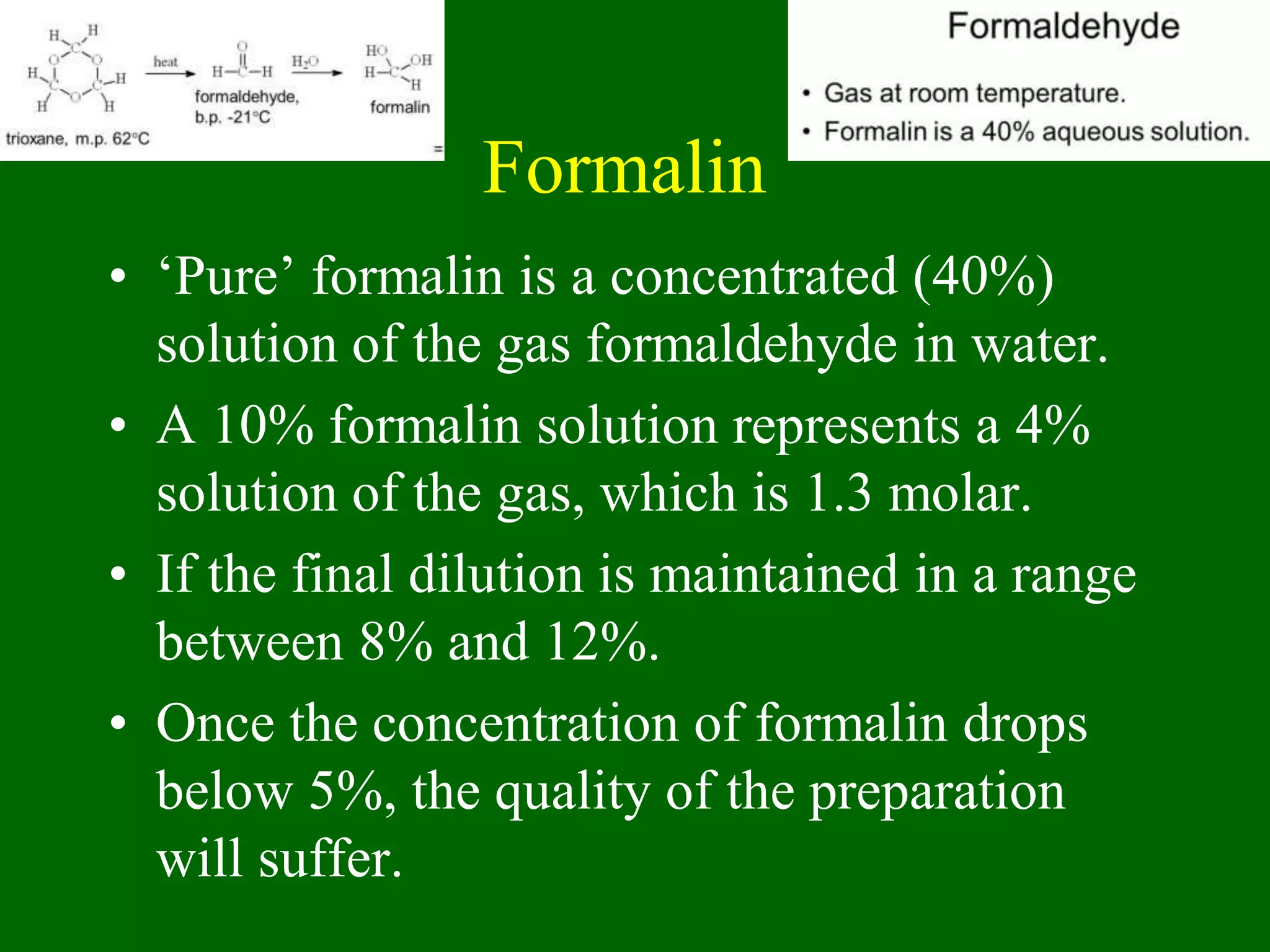
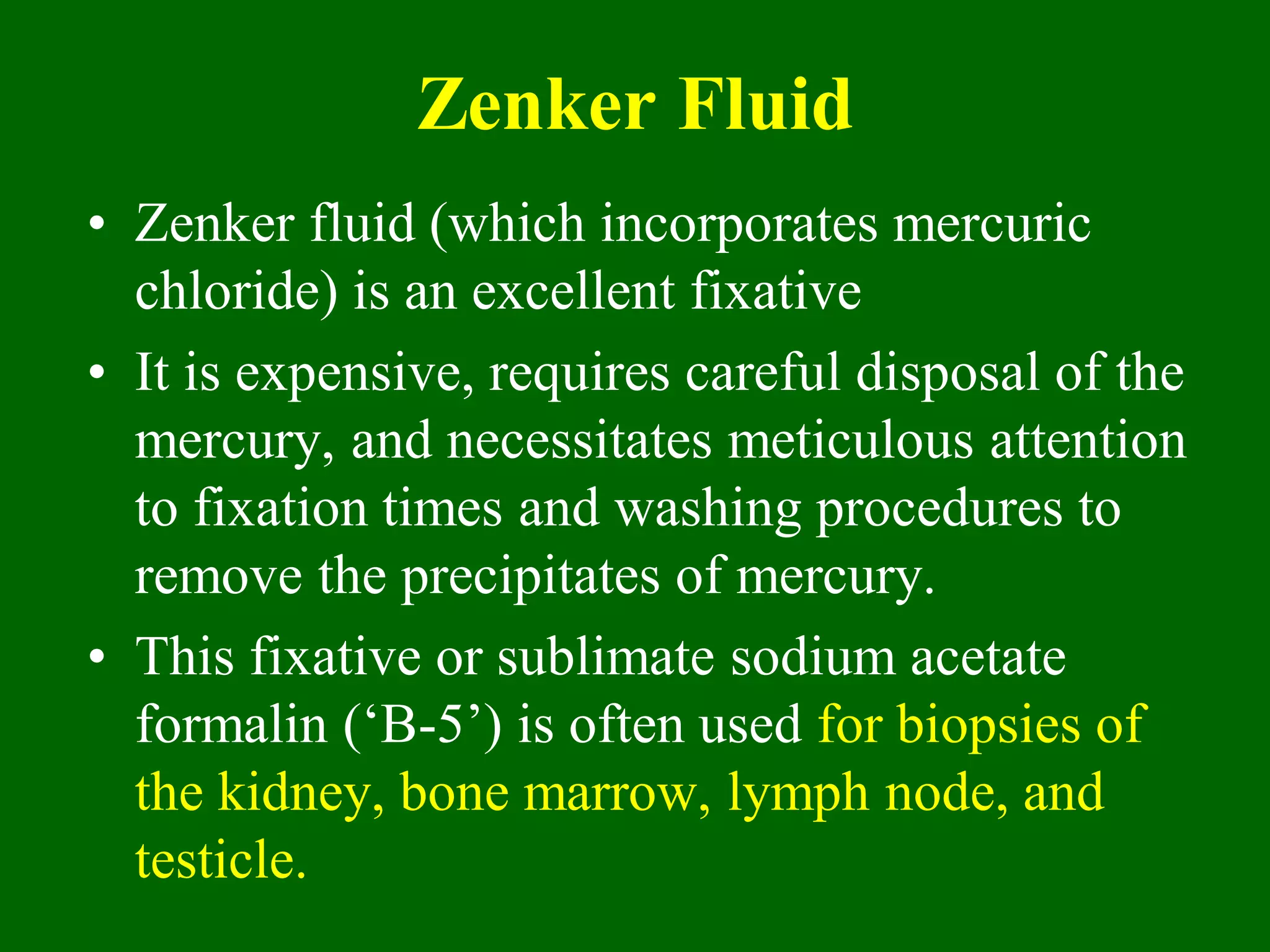
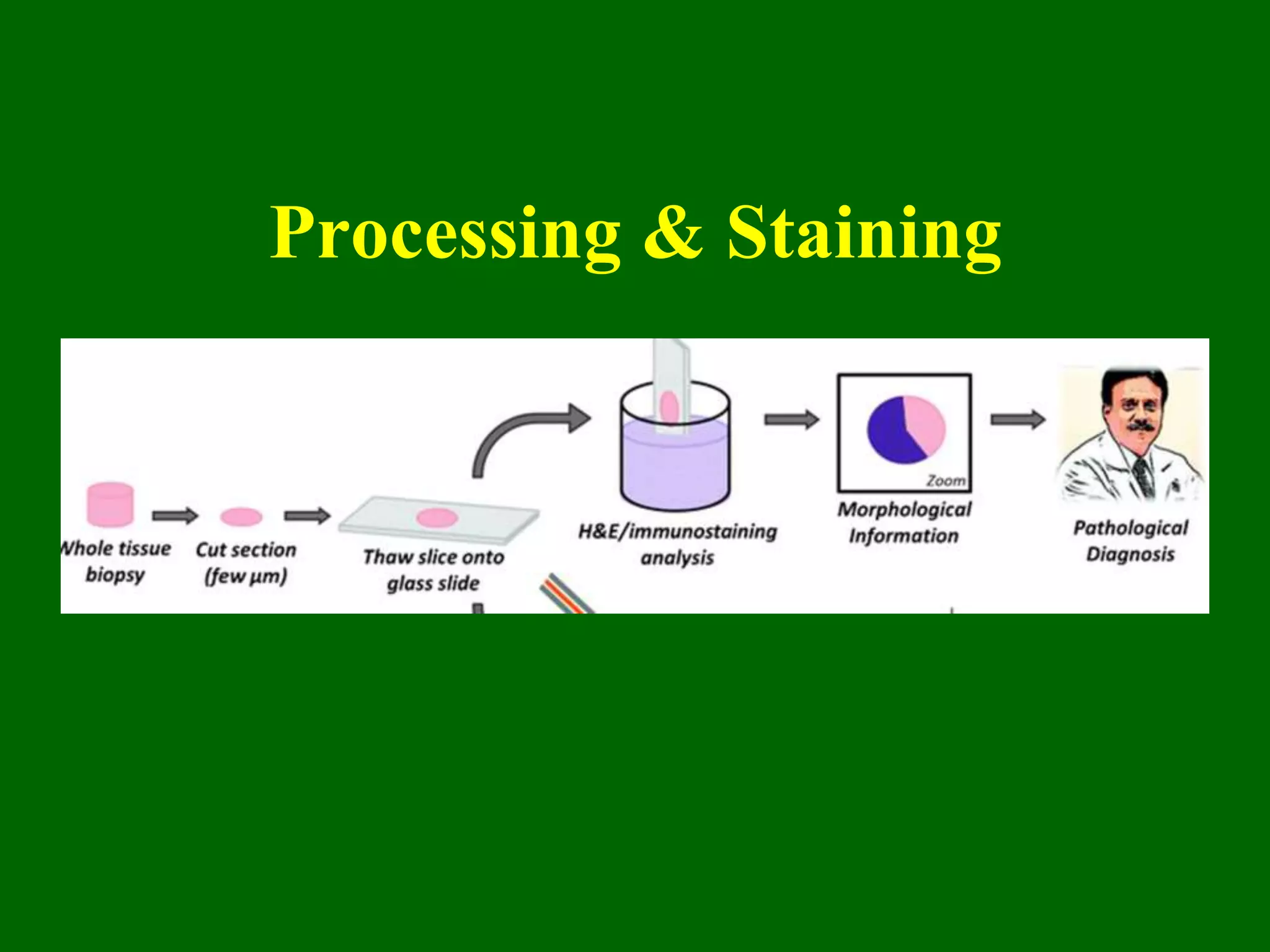
1) Tissue specimens are fixed in formalin to prevent deterioration. They then undergo dehydration, clearing, infiltration with paraffin wax, and embedding to allow sectioning.
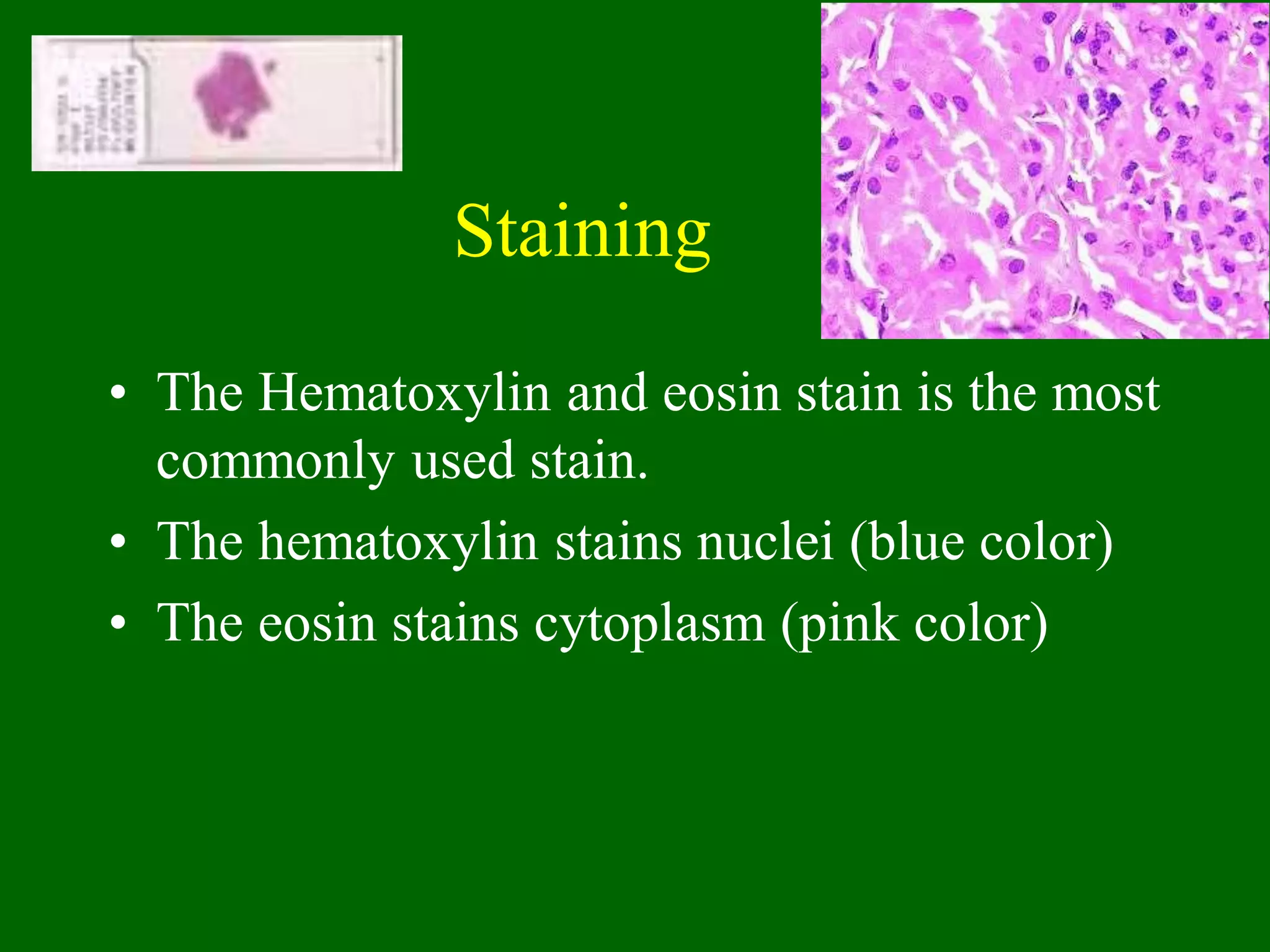
2) Sections are cut on a microtome and stained, usually with hematoxylin and eosin, with hematoxylin staining nuclei blue and eosin staining cytoplasm pink.
3) Care must be taken to properly handle, sample, and process tissues to avoid contamination and allow accurate histological examination.