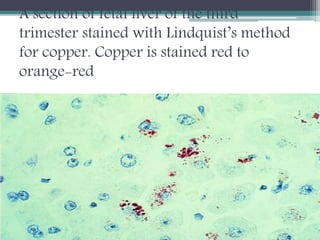
This document discusses different types of pigments found in tissues. It describes endogenous pigments such as hematogenous pigments (hemosiderin, hemoglobin), bile pigments, porphyrins, melanin, lipofuscin and chromaffin. Hemosiderin contains stored iron and appears as yellow-brown granules. Various stains can demonstrate iron, including Perl's Prussian blue. Hemoglobin transports oxygen and is stained blue with patent blue. Bile pigments are breakdown products of hemoglobin transported to the liver and gallbladder. Lipofuscin is an oxidation product that accumulates with age in various tissues. The document also discusses exogenous pigments and methods for demonstrating various endogenous pigments