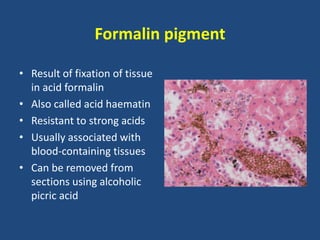
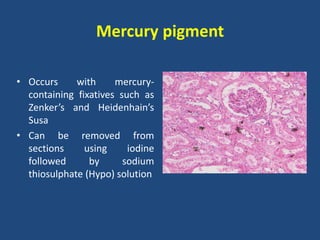
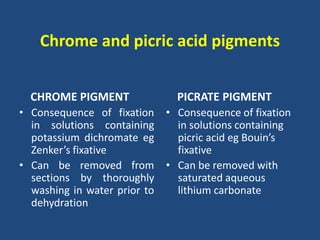
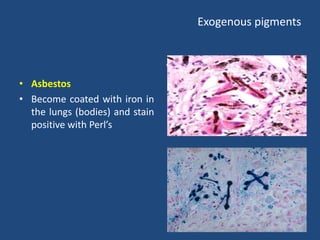
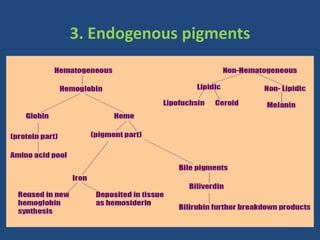
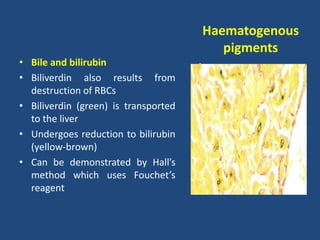
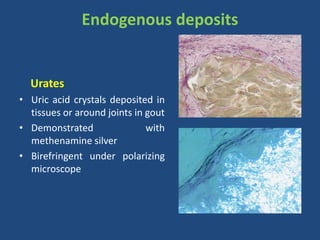
The document classifies pigments and minerals into three categories: artefact, exogenous, and endogenous. It details the characteristics, origins, and staining methods for each type, including various pigments associated with tissue fixation and those derived from the body or environment. Additionally, it describes techniques for studying inorganic substances and the potential pathological deposits of minerals in tissues.