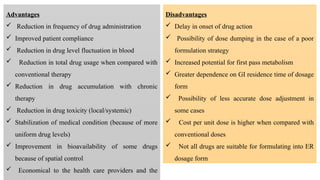
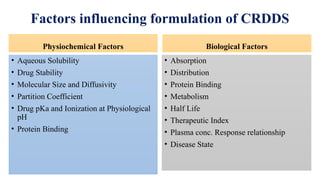
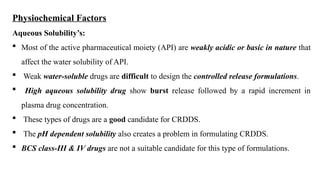
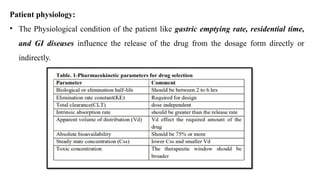
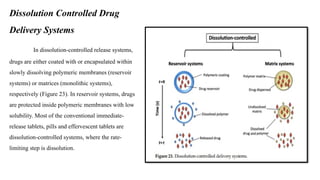
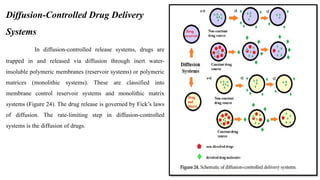
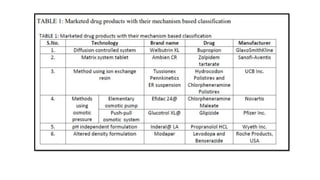
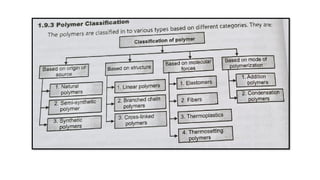
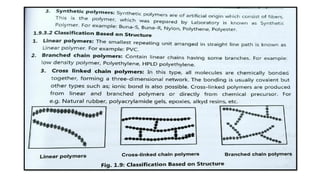
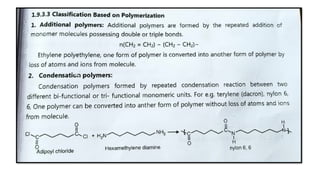
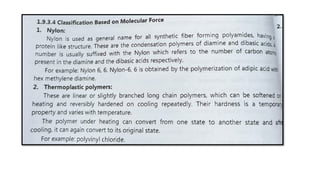
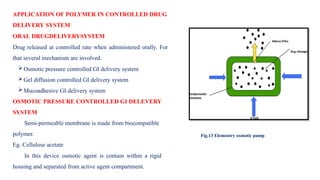
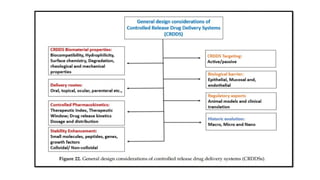
The document discusses controlled release drug delivery systems (CRDDS), which deliver drugs at predetermined rates to optimize therapeutic efficacy while minimizing side effects. It highlights the advantages of CRDDS, such as improved patient compliance and reduced drug accumulation, compared to conventional therapies, while also noting challenges like potential dose dumping and higher costs. Various factors influencing the formulation and effectiveness of CRDDS, including drug properties, physiological factors, and the role of polymers in drug delivery systems, are extensively covered.