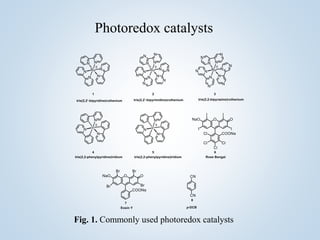
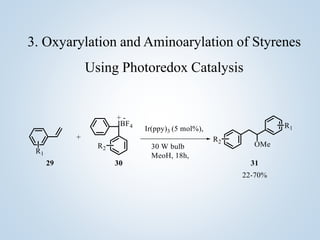
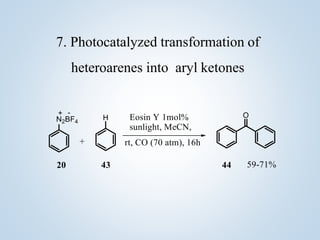
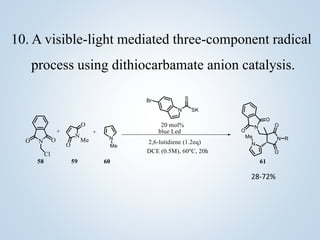
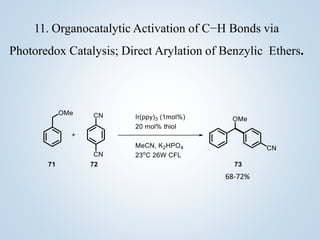
This document summarizes Sameer Hussain's thesis project on the construction of carbon-carbon bonds via photogenerated intermediates under the supervision of Prof. Ravi P. Singh. It introduces various photochemical and photocatalytic methods for carbon-carbon bond formation using reactive intermediates like radicals, carbenes, and biradicals generated with photoredox catalysts like eosin Y and ruthenium complexes under visible light. Several examples of aryl-carbon bond formation via ipso substitution and cross-coupling reactions conducted with high yields between 68-92% are summarized. The document concludes by anticipating broad applications of these photoredox reactions and discussing future research perspectives in the field.