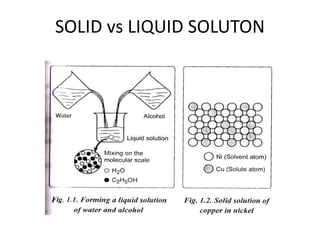
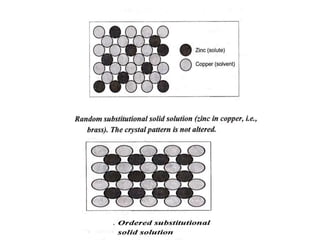
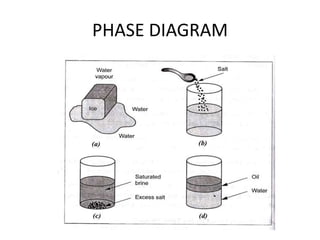
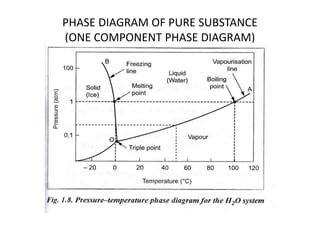
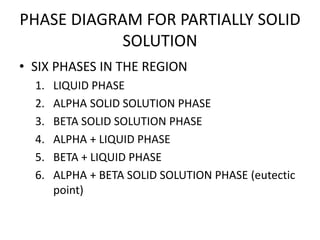
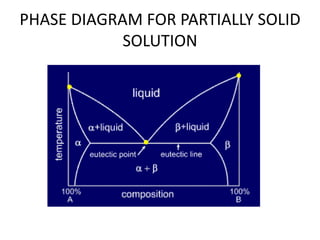
This document discusses solid solutions and phase diagrams in engineering metallurgy. It defines key terms like alloy, solute, solvent and phase. It explains that solid solutions occur when elements are atomically dispersed in a single crystal structure, following Hume-Rothery rules regarding size, structure, valence and electronegativity. Phase diagrams graphically represent phases present at various temperatures, pressures and compositions, and the Gibbs phase rule determines the maximum number of phases based on components and system. Examples are given of phase diagrams for pure substances and alloys with partially solid solutions.