Embed presentation
Download to read offline





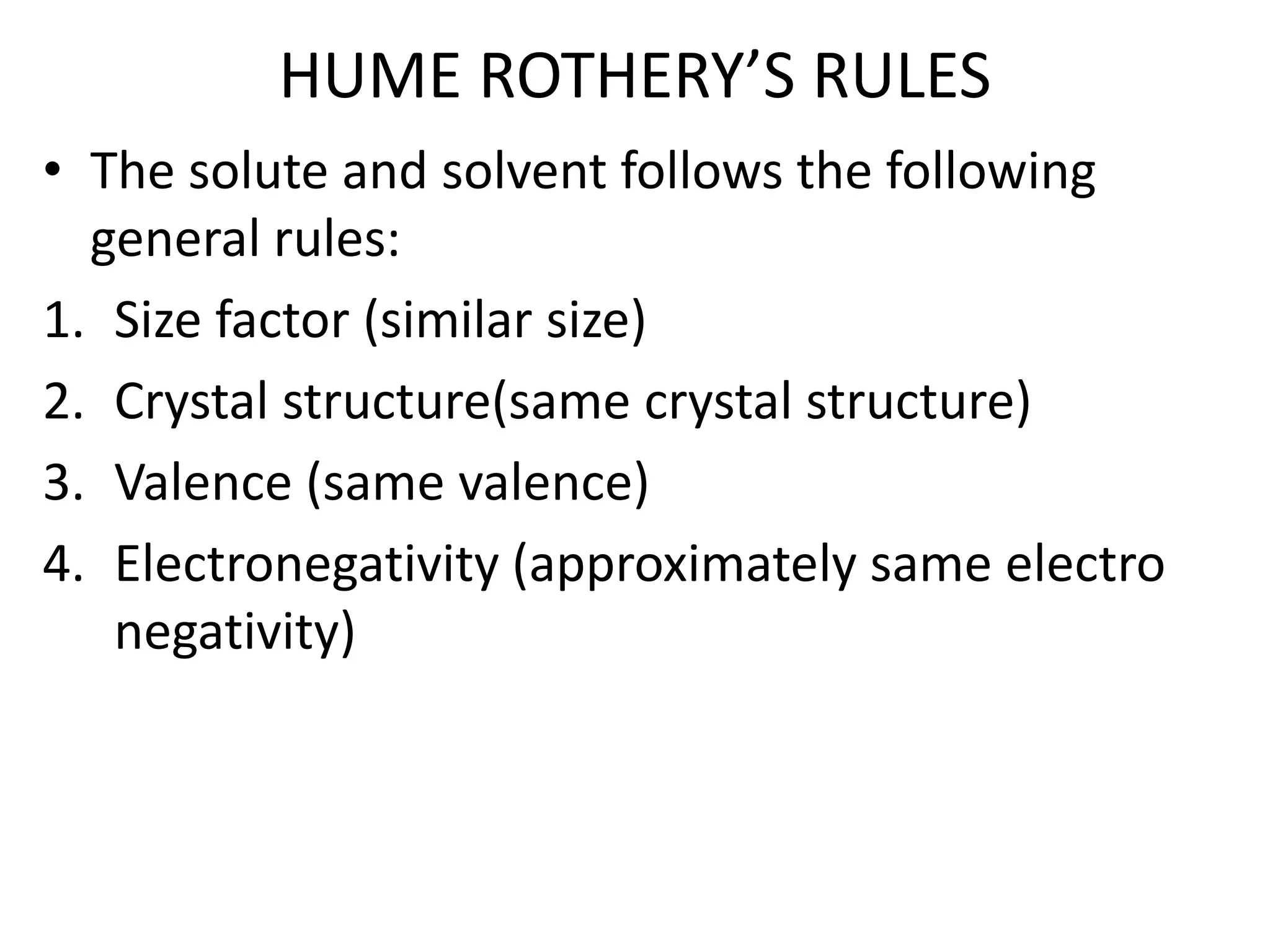



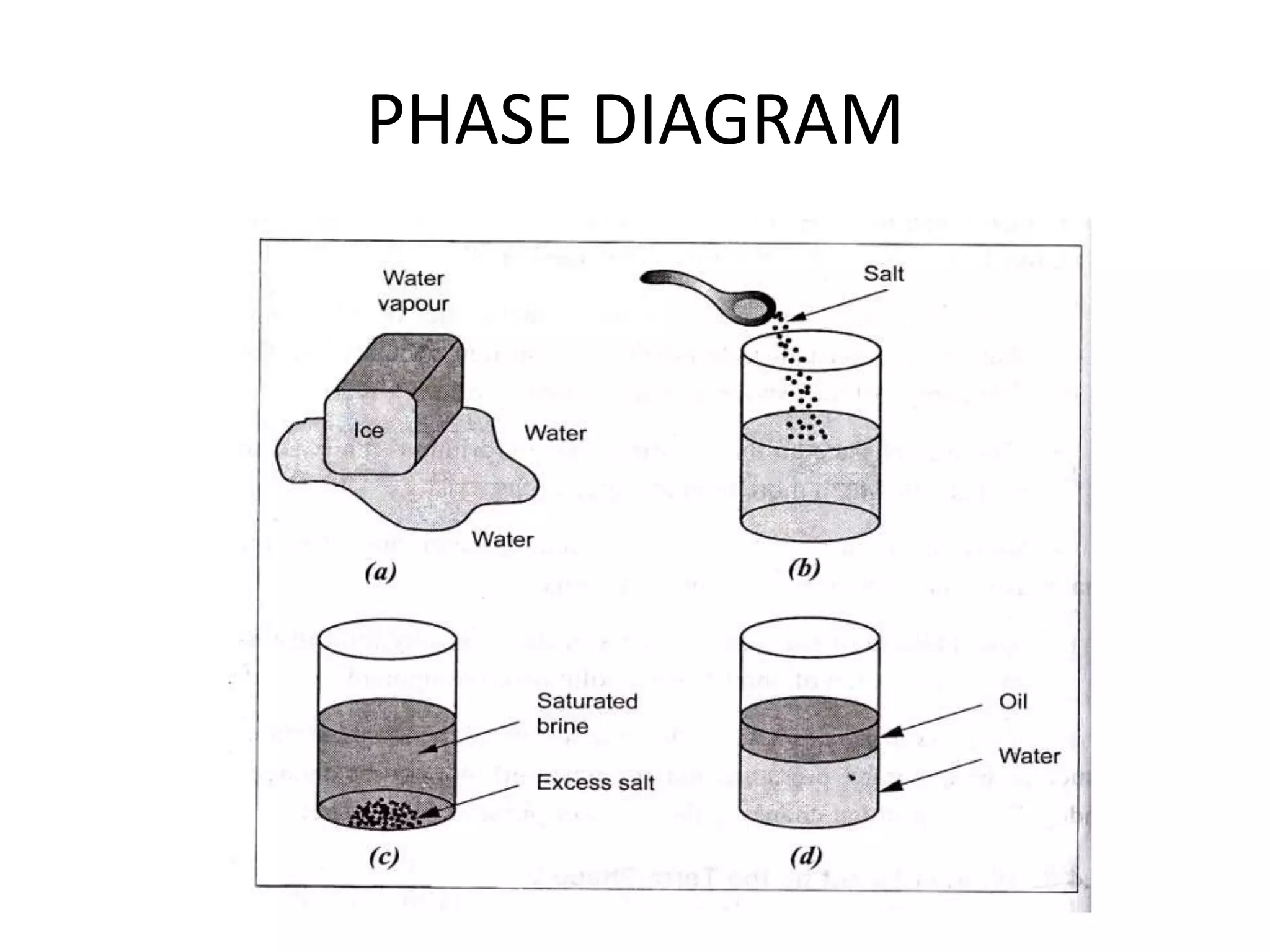





This document discusses alloys and phase diagrams, which are important concepts in metallurgy and materials engineering. It covers the constitution of alloys including solid solutions, Hume-Rothery's rules for solid solubility, and the different types of solid solutions. The document also discusses phase diagrams, including one-component phase diagrams for pure substances and phase diagrams for partially solid solutions that can have up to six phases. Phase diagrams are critical tools for understanding phase transformations and equilibrium conditions in alloy systems.













