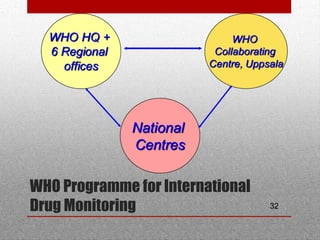
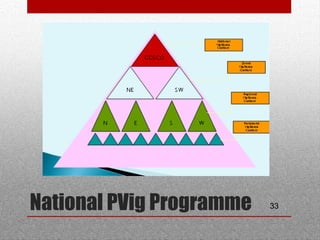
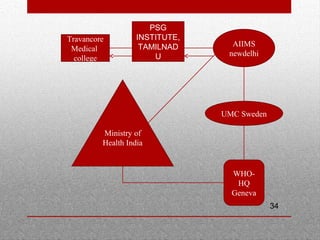
This document discusses pharmacovigilance and adverse drug reactions. It defines an adverse drug reaction and explains that the goal of pharmacovigilance is to detect, understand, and prevent such reactions. It outlines who the key partners are in pharmacovigilance efforts, including governments, health professionals, patients, and WHO. The document also explains the importance of pharmacovigilance, citing examples like the thalidomide disaster and significant costs of adverse drug reactions. It emphasizes that pharmacovigilance is needed to promote rational drug use and ensure public safety and confidence in medical treatments.