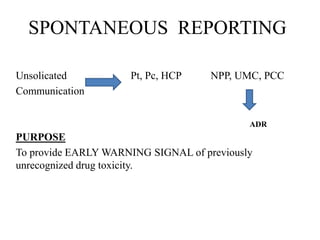
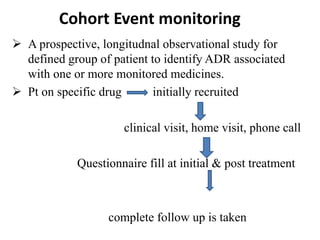
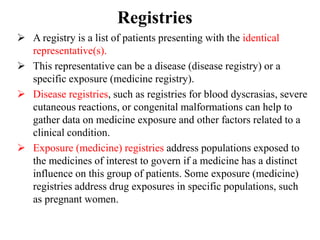
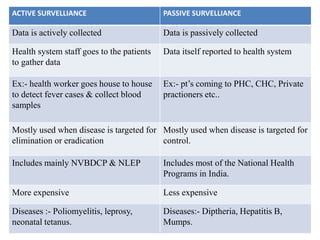
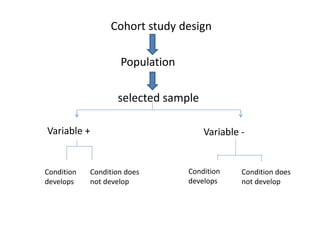
This document discusses different methods for pharmacovigilance including spontaneous reporting, active surveillance, sentinel sites, cohort event monitoring, and registries. Spontaneous reporting involves voluntary reporting of adverse drug reactions by healthcare professionals and can provide early signals of unrecognized toxicity. Active surveillance methods like cohort event monitoring and sentinel sites pursue to determine a specific number of adverse events through continuous organized monitoring.