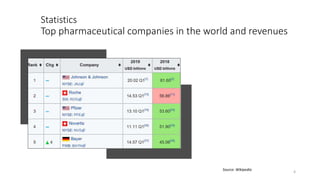
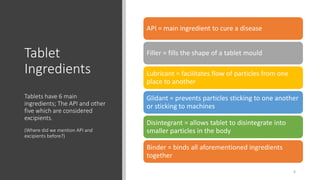
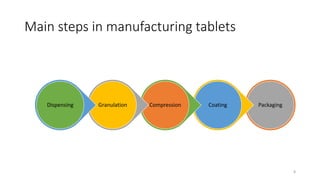
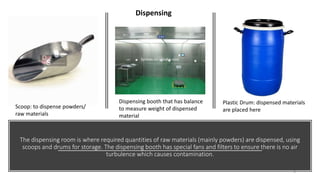
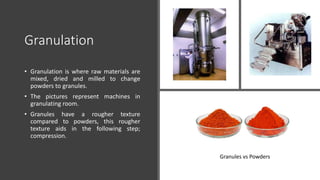
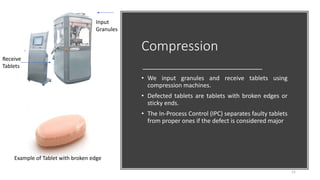
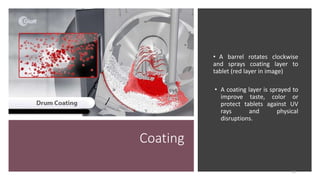
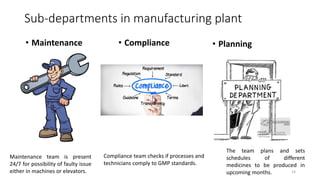
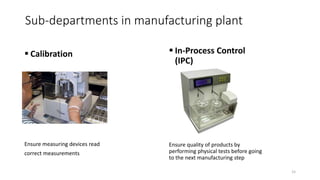
The document provides an overview of the pharmaceutical industry, focusing on the manufacturing processes of tablets, including key ingredients, such as active pharmaceutical ingredients (API) and excipients. It outlines the steps involved in tablet production, from dispensing to packaging, and describes the roles of various sub-departments within a pharmaceutical plant. Key concepts such as good manufacturing practices (GMP) and in-process control (IPC) are also discussed to highlight quality assurance in the industry.