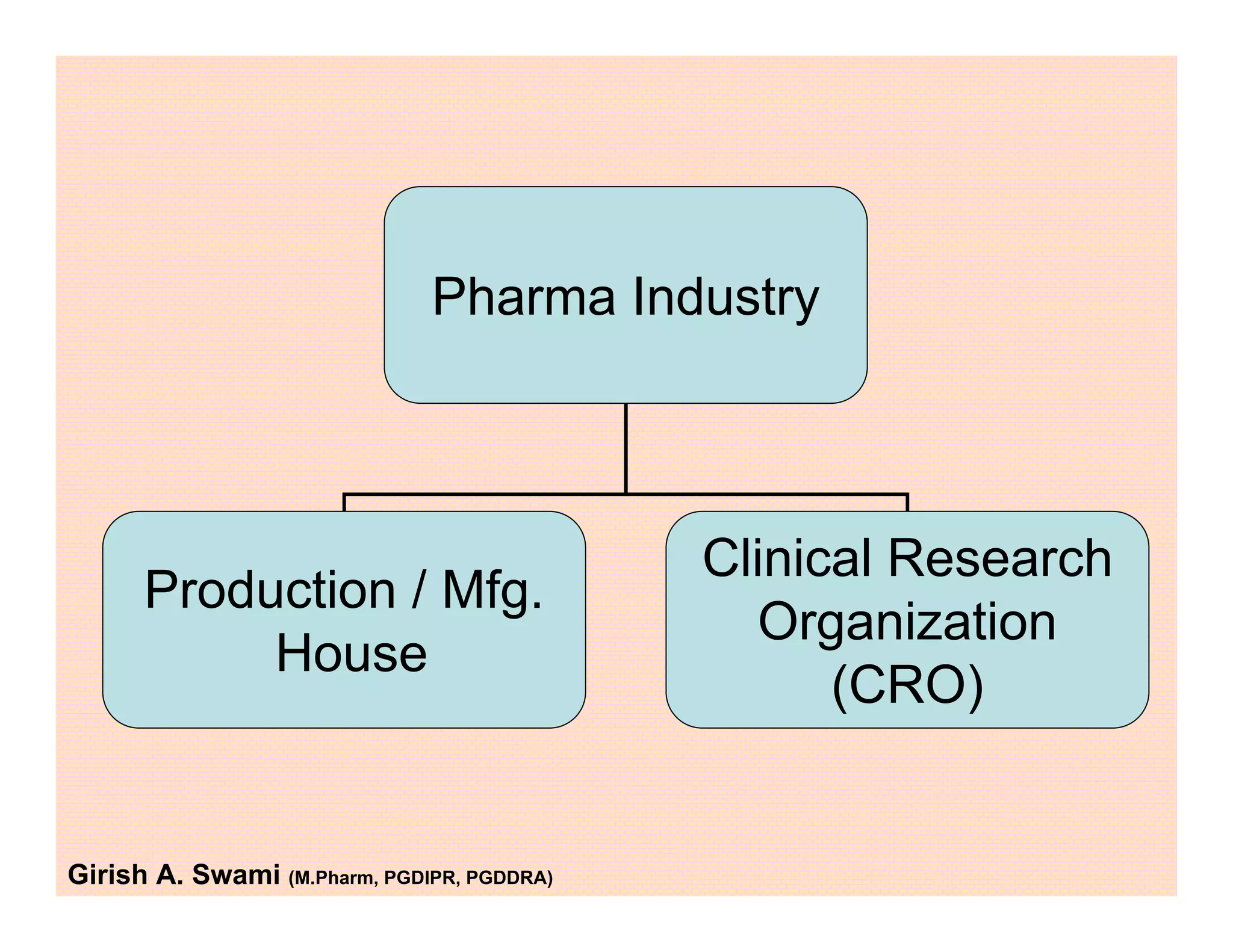
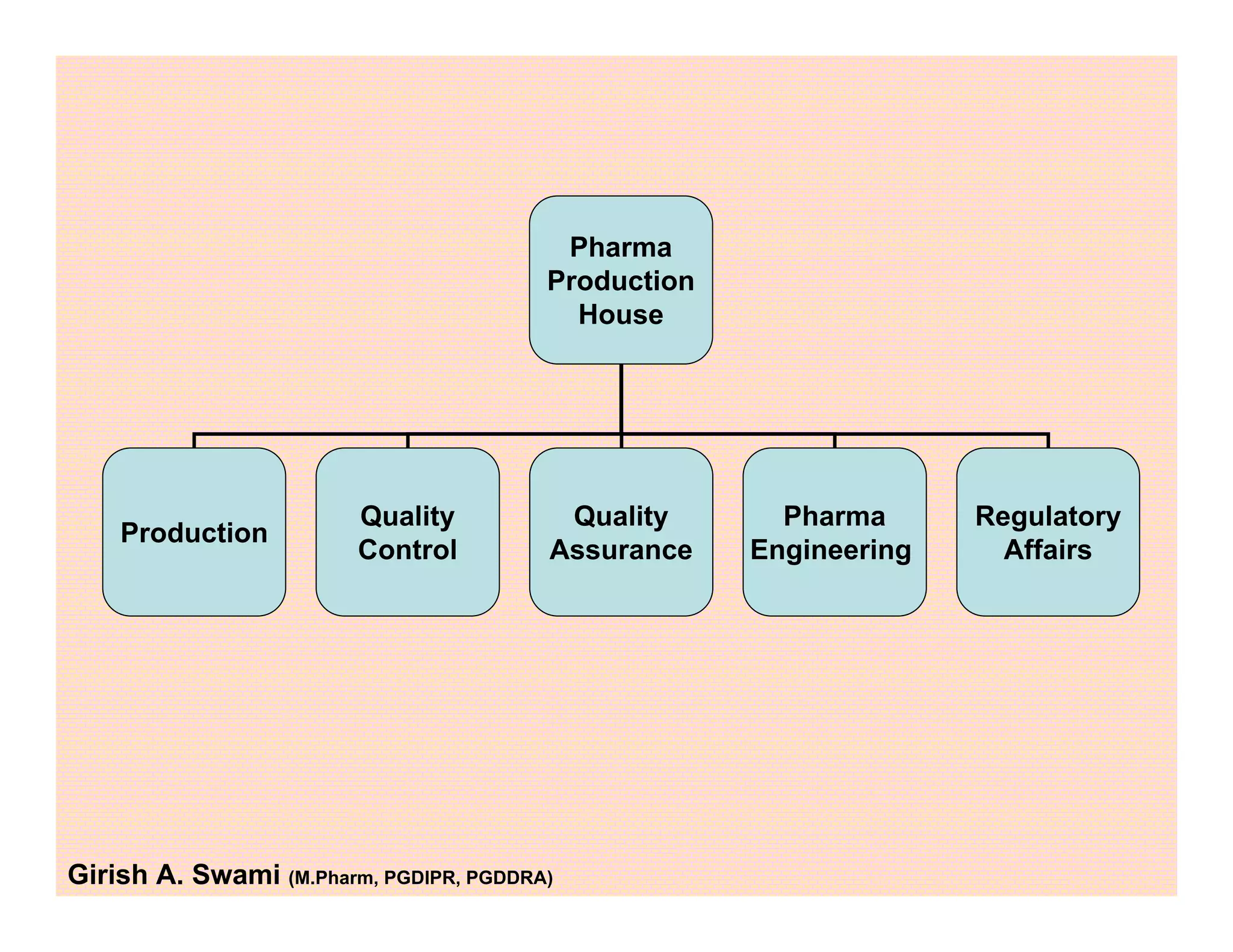
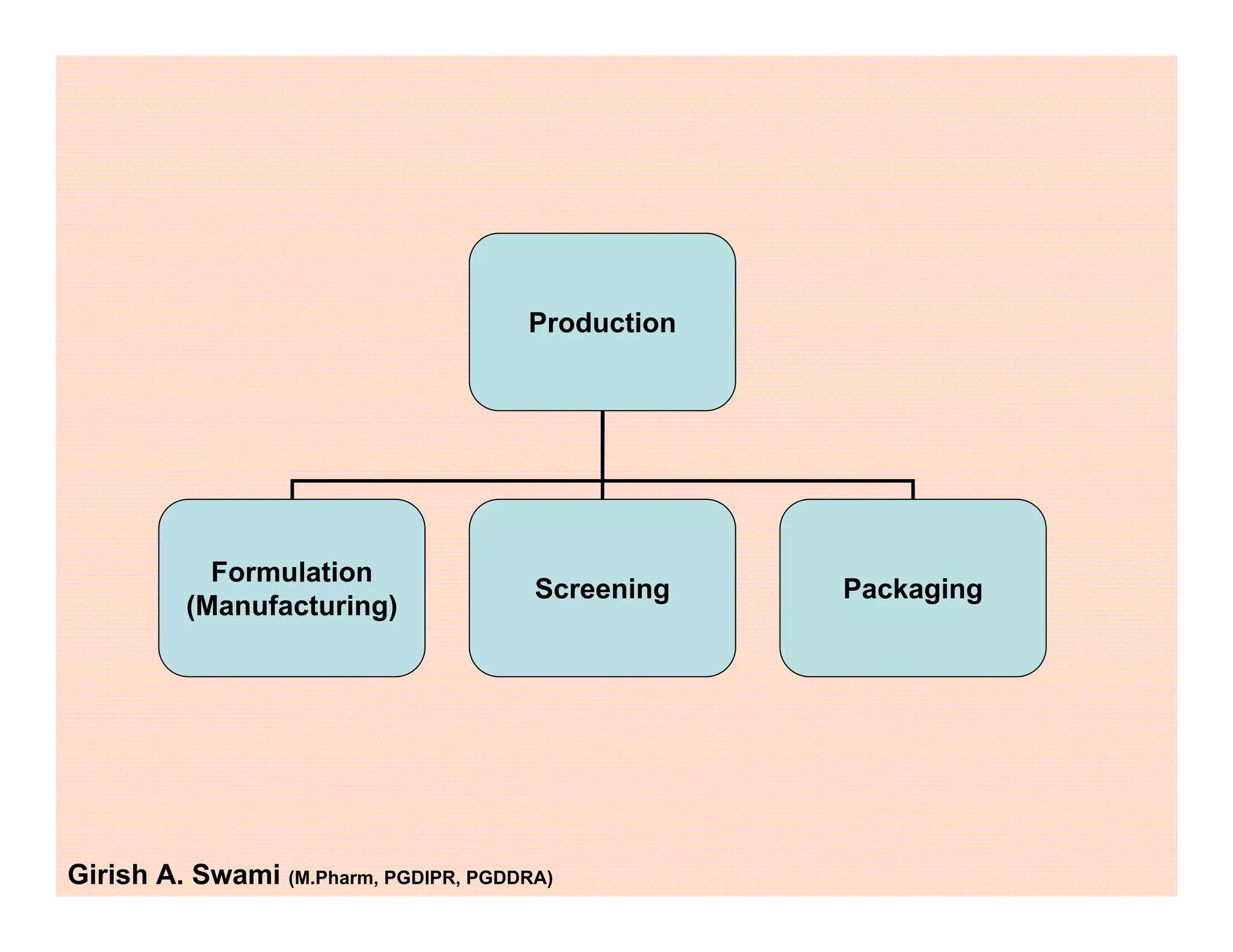
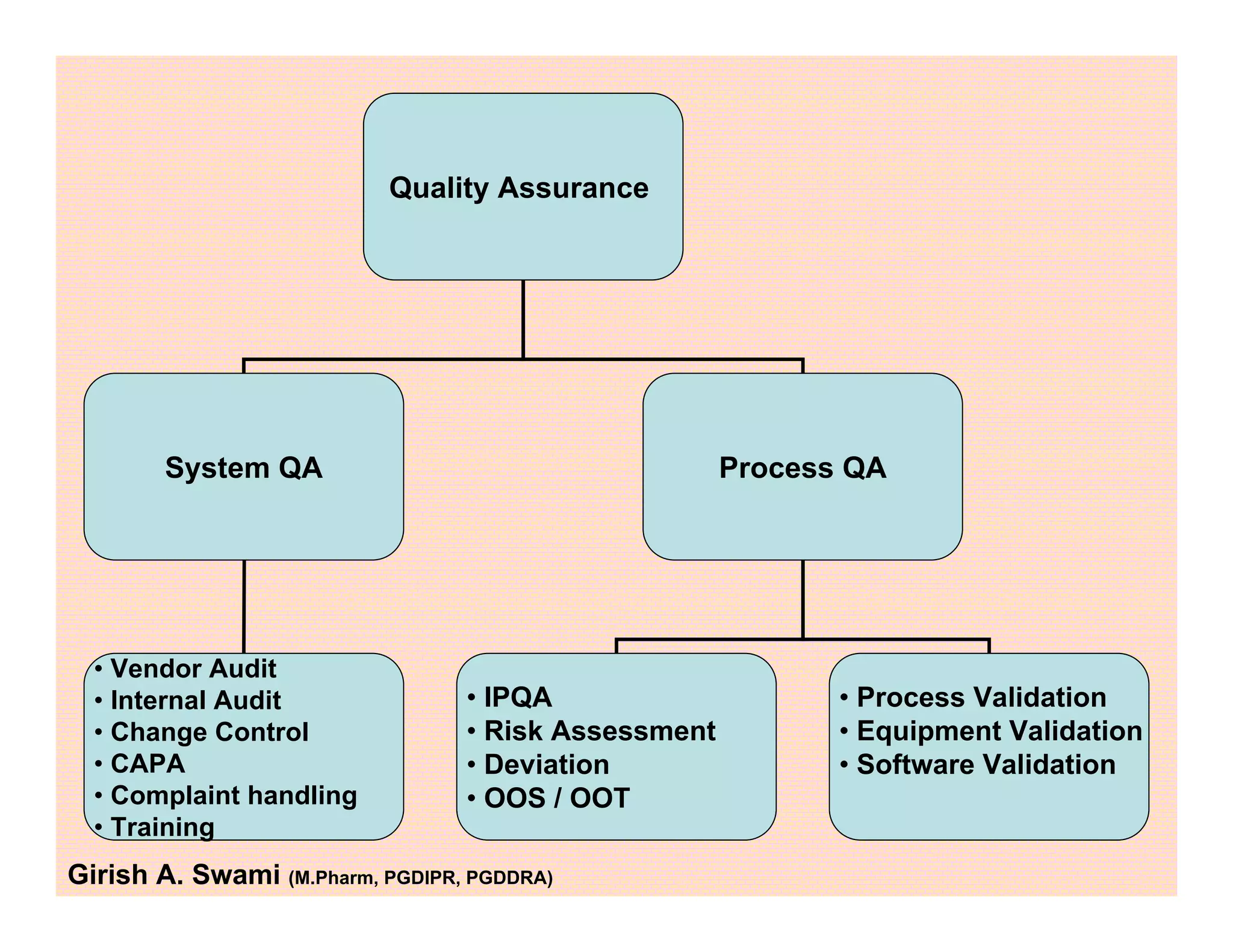
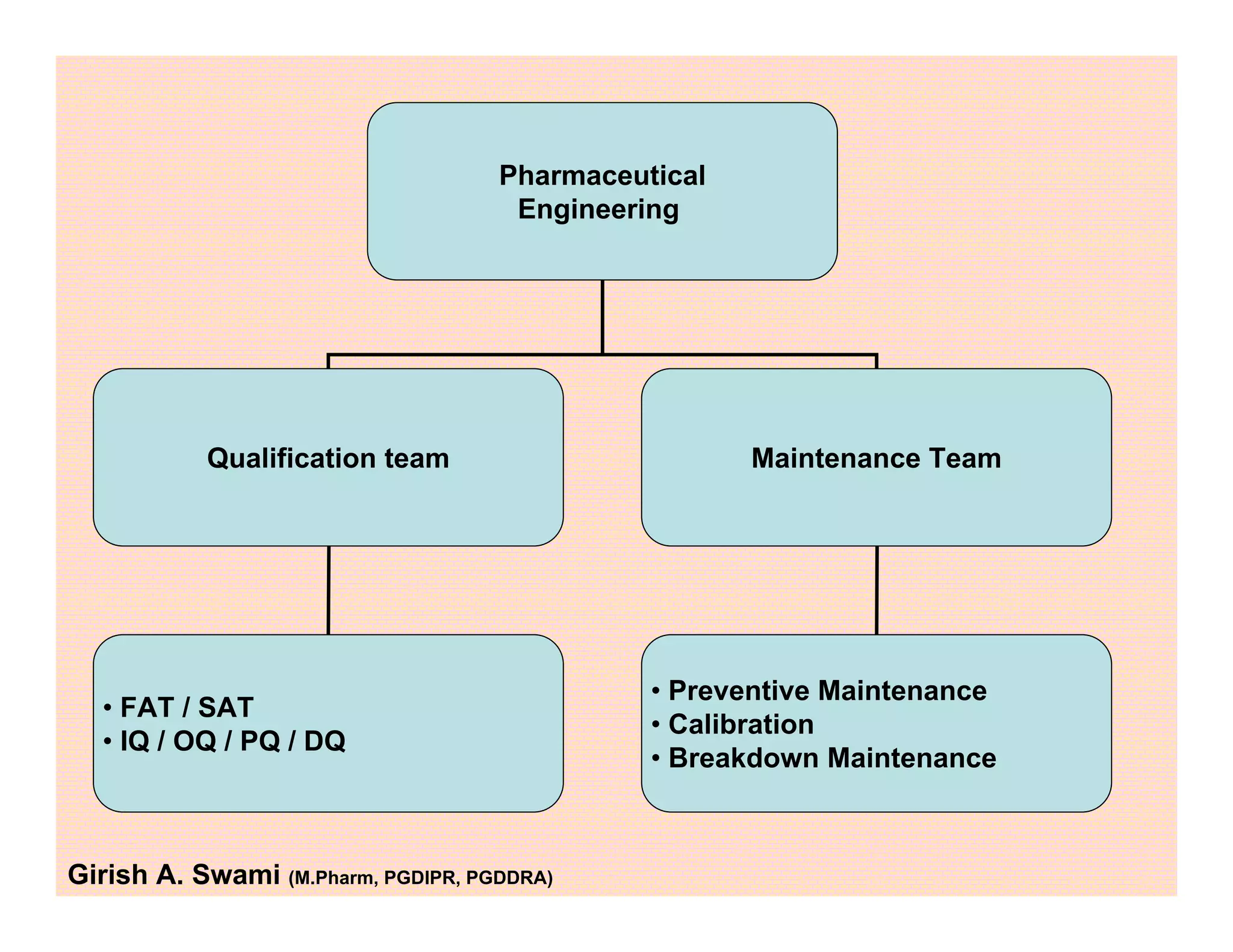
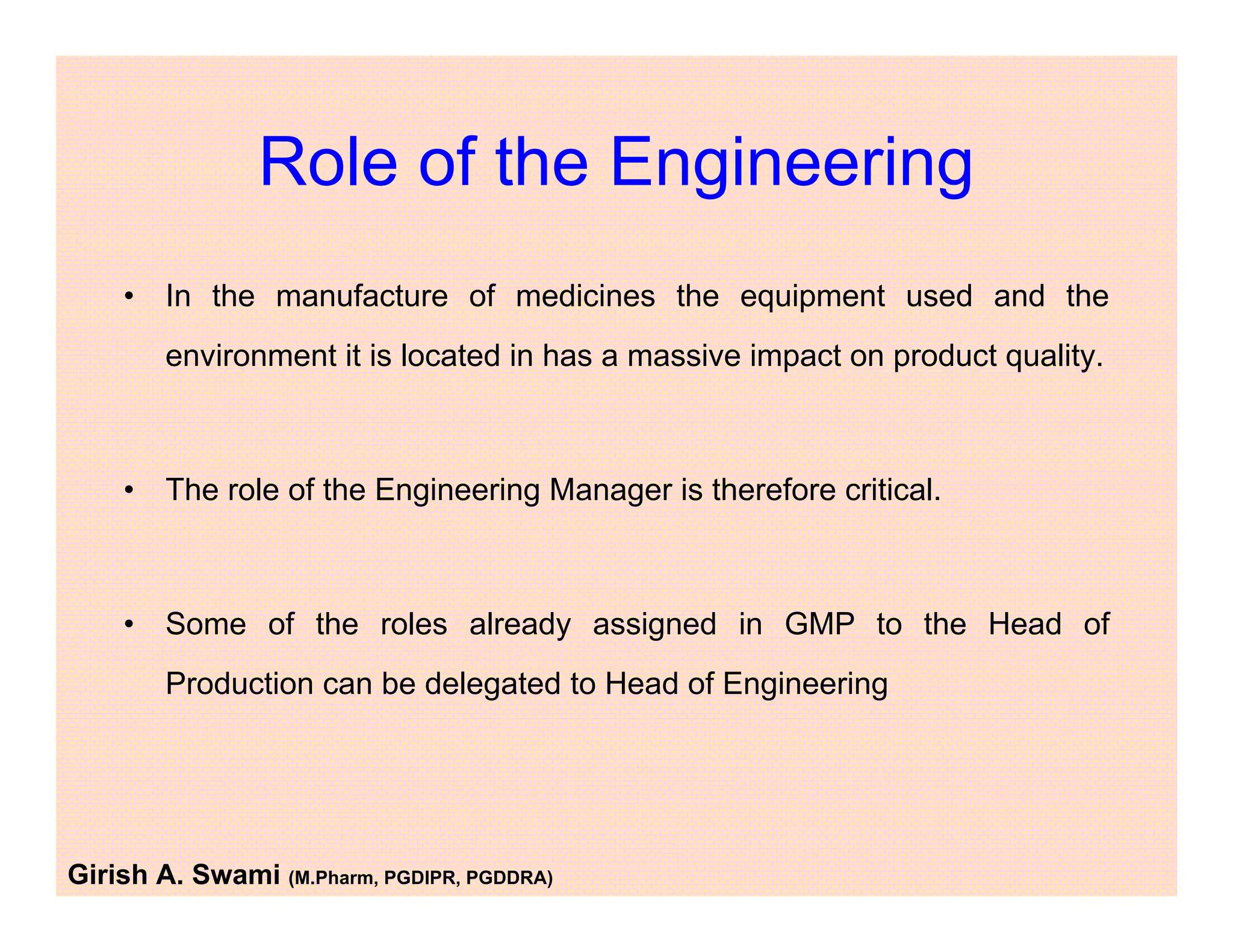
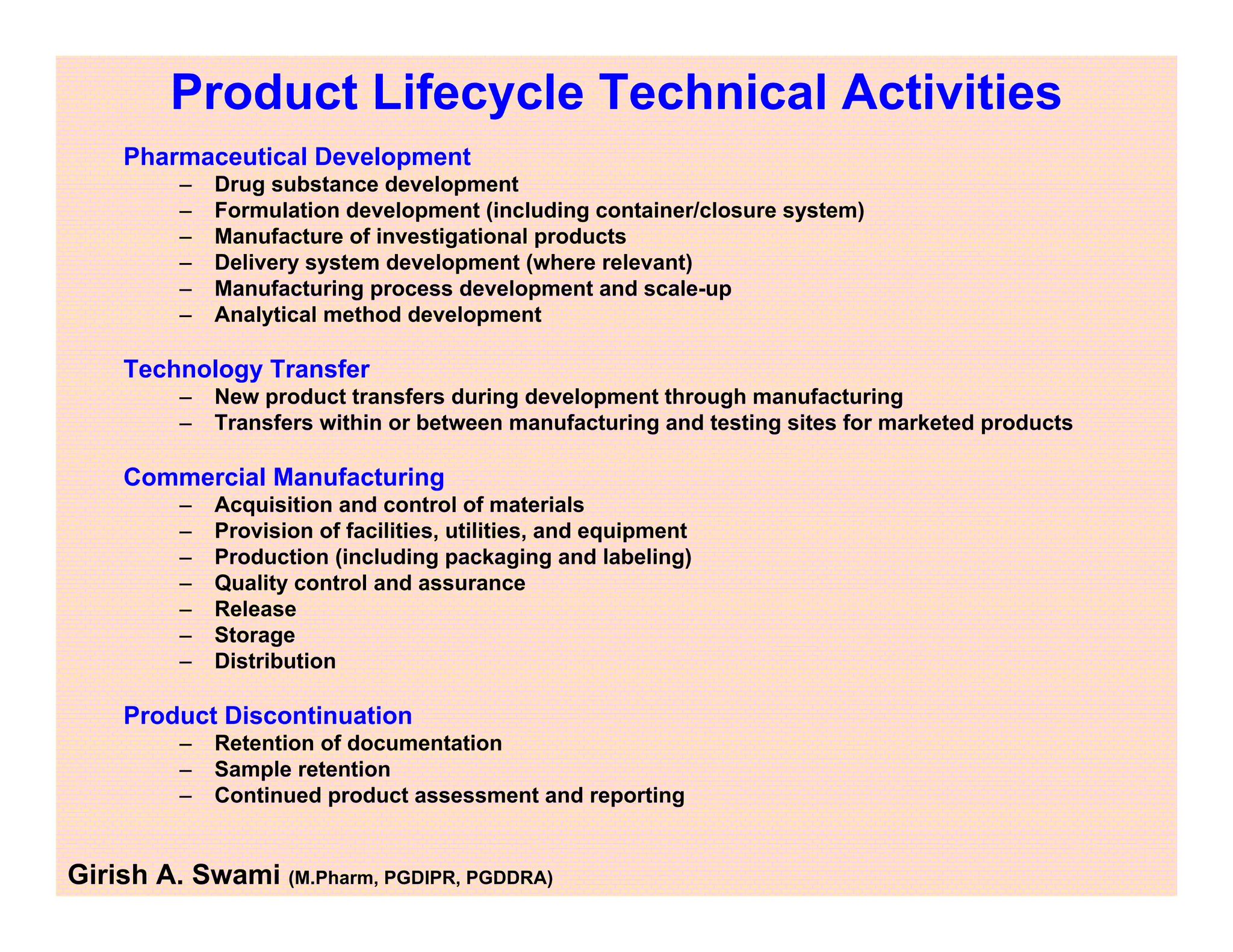
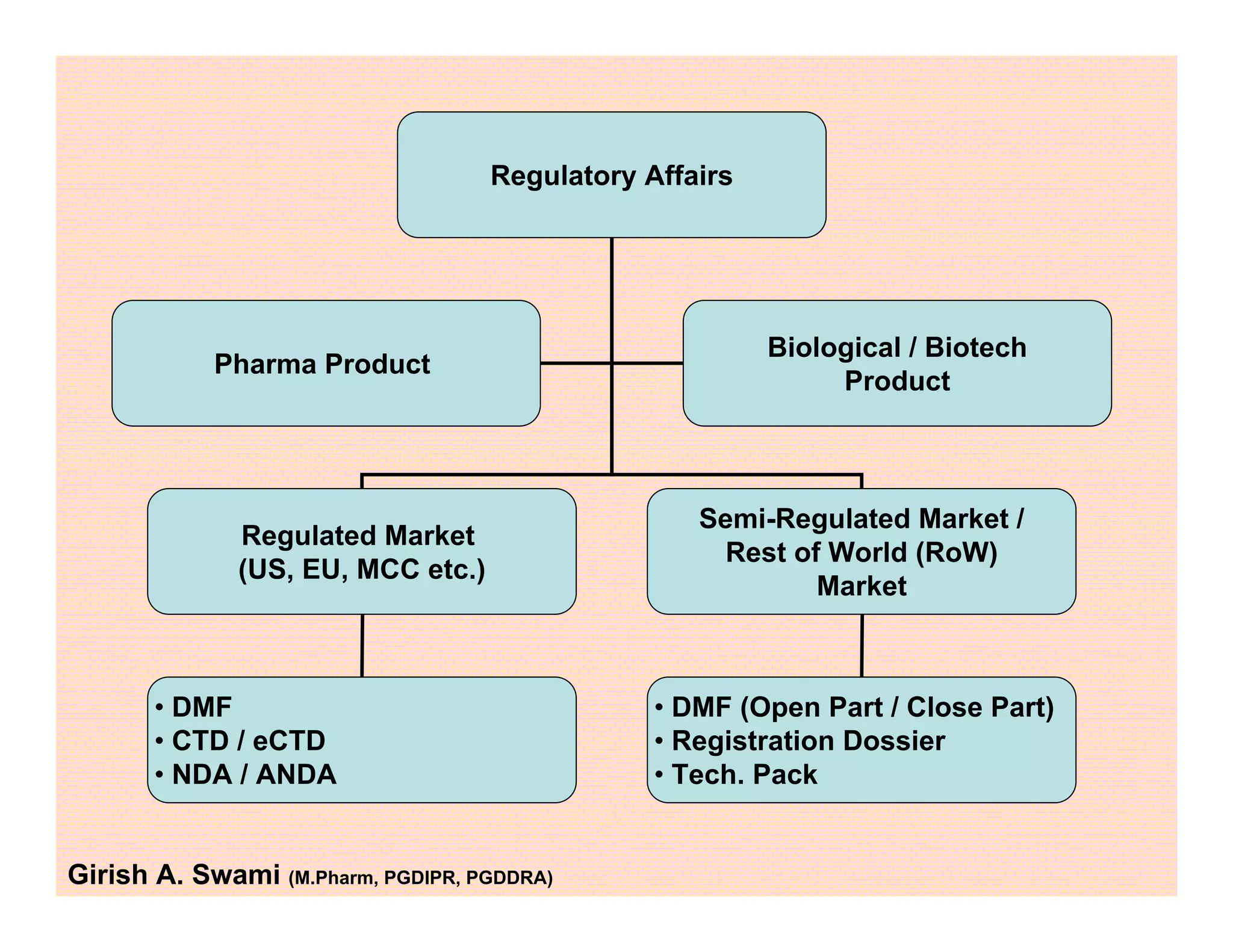
This document provides an overview of the key departments and functions within the pharmaceutical industry. It describes the roles of production, quality control, quality assurance, engineering, regulatory affairs, and clinical research organizations. It also outlines the responsibilities of key personnel like heads of production and quality control, as well as the qualified person. The document emphasizes that effective quality management systems are important for product realization, process control, and continual improvement.







![The Qualified Person [QP]
(EU GMP)
• Products manufactured within EU – a QP must ensure that each batch
was produced and tested according to GMP and Licensed conditions
• Products manufactured outside the EU – a QP must ensure that each
imported batch has undergone, in the importing country re-testing and
evaluation
• A QP must sign a register to state that the above has been performed
satisfactorily before product is released (QP certification)
• Product Quality Review performed in a timely manner and accurate
(EU GMP 1.5)
• QP have authority to certify a batch for release.
Girish A. Swami (M.Pharm, PGDIPR, PGDDRA)](https://image.slidesharecdn.com/introductiontopharmaindustry-160413050611/75/Introduction-to-pharma-industry-11-2048.jpg)





















![What to do
[Knowledge, Skills and Abilities]
• Industry Knowledge/ Experience: Knowledge of applicable FDA regulatory
standards and requirements.
• Communication Skills: Advanced level of written and verbal communication
skills with the ability to read, analyze and interpret general business
periodicals, professional journals, technical procedures or governmental
regulations. Ability to write reports, business correspondence and
procedure manuals. Ability to effectively present information to plant
employees and the general public and lead in a team environment.
• Reasoning Ability: Ability to solve practical problems and deal with a
variety of concrete variables in situations where only limited standardization
exists. Ability to interpret a variety of instructions furnished in written, oral,
diagram or schedule form.
• Leadership: Effectively manage competing priorities, adaptable to
change, demonstrated ability to accept additional responsibilities and manage
tasks through teamwork.
Girish A. Swami (M.Pharm, PGDIPR, PGDDRA)](https://image.slidesharecdn.com/introductiontopharmaindustry-160413050611/75/Introduction-to-pharma-industry-40-2048.jpg)
