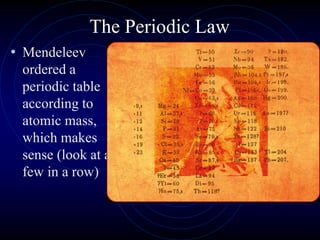
1. Dimitri Mendeleev was the first to publish an organized periodic table and predicted the properties of undiscovered elements.
2. Henry Moseley later arranged the periodic table by atomic number, which is the modern version.
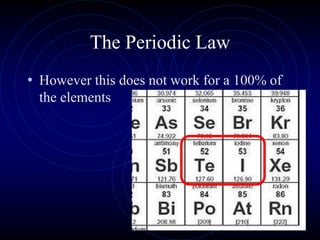
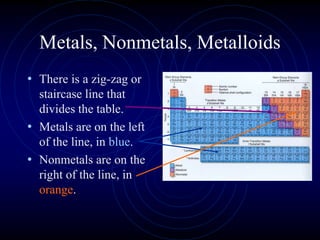
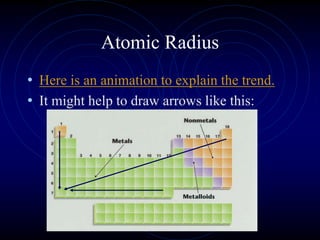
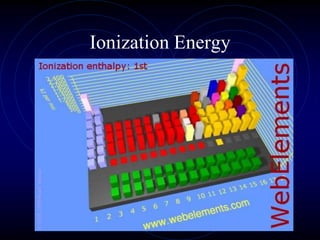
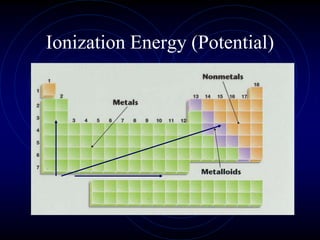
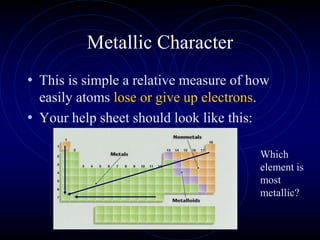
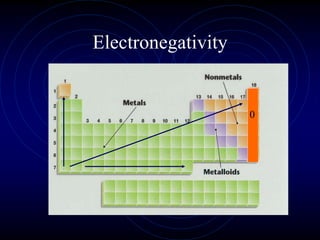
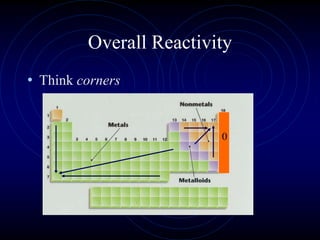
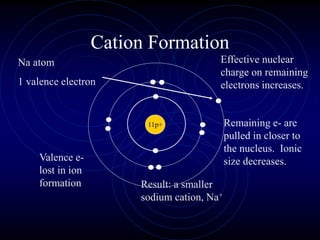
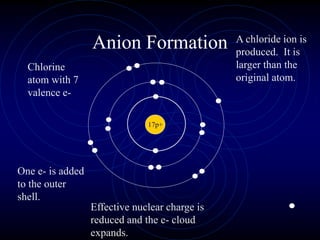
3. The periodic law states that when elements are arranged by atomic number, they display a repeating pattern of chemical and physical properties.