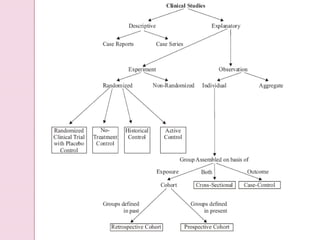
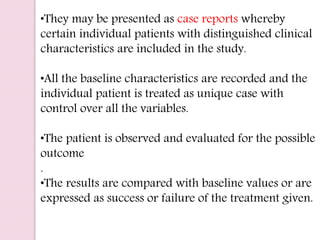
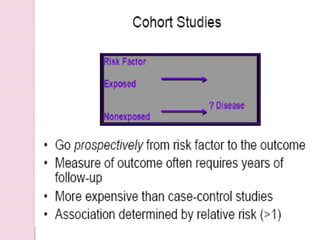
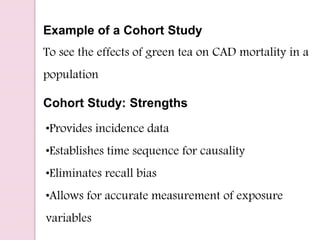
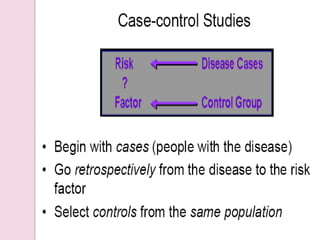
Post-marketing surveillance is important to identify adverse drug reactions that were not detected in pre-market clinical trials due to limited sample sizes. There are several methods used for post-marketing surveillance including spontaneous reporting, cohort studies, and case-control studies. These methods help monitor drug safety once a drug is on the market and exposed to a more diverse population and conditions compared to clinical trials. Post-marketing surveillance is especially important for detecting rare or long-term adverse effects.




![•Once the drug enters the market, it will be utilized
by many more patients having other co-morbidity
and co-existing diseases in addition to the disease for
which the drug is indicated and licensed.
No fixed duration/Patient population
Starts immediately after marketing
Report all ADRs
Help to detect
Rare ADRs
Drug interaction
Also new uses for drugs[sometimes called Phase
V]](https://image.slidesharecdn.com/3-170729060418/85/post-marketing-surveillance-methods-5-320.jpg)