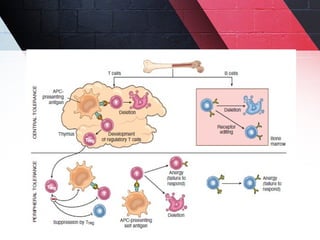
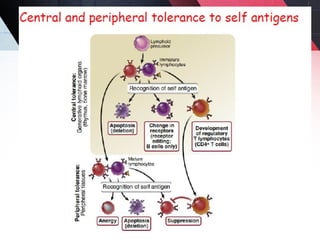
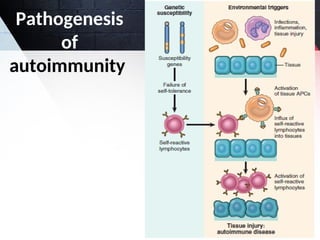
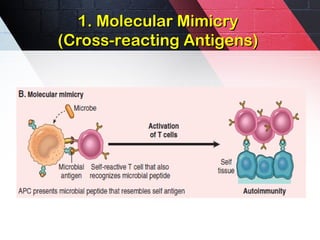
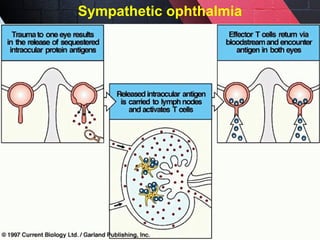
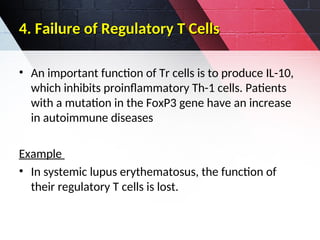
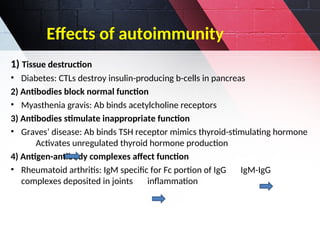
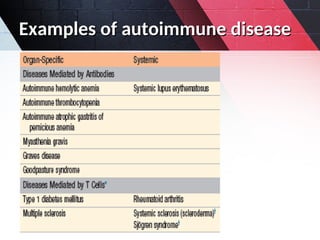
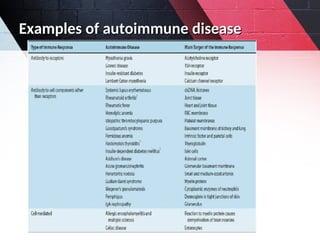
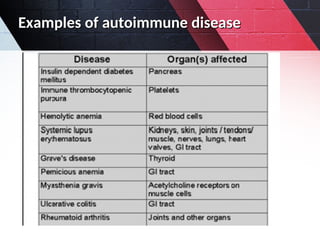
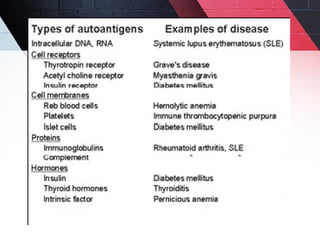
The document discusses autoimmunity, focusing on T and B cell tolerance and the mechanisms that contribute to autoimmune diseases. It outlines two types of immunological tolerance: central and peripheral, as well as the pathogenesis involving factors such as molecular mimicry and environmental triggers. Additionally, it highlights the role of genetic predisposition and provides examples of various autoimmune disorders.