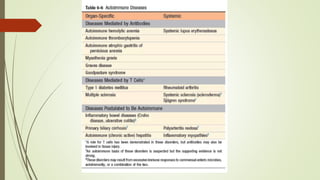
The document provides an overview of autoimmunity, detailing various mechanisms such as central and peripheral tolerance, and the development of autoimmune diseases such as rheumatoid arthritis, Sjögren’s syndrome, and systemic sclerosis. It discusses the role of genetic susceptibility, environmental triggers, and the pathological processes involved, outlining clinical features and diagnosis for each condition. Additionally, it highlights the importance of immune regulatory mechanisms and the consequences of their failure in autoimmune pathogenesis.