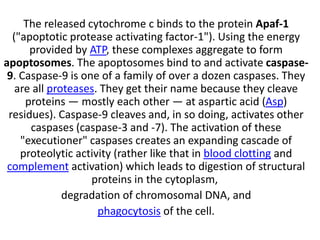
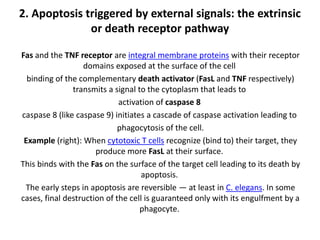
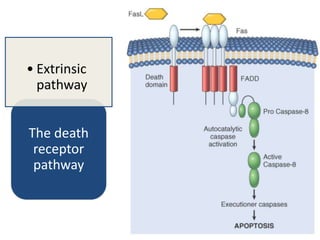
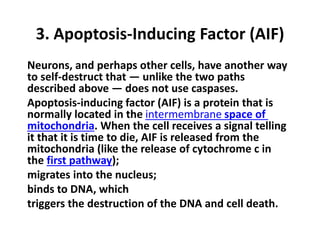
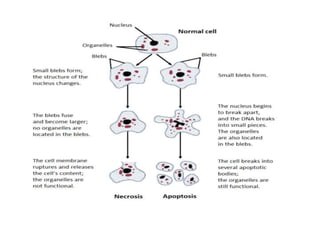
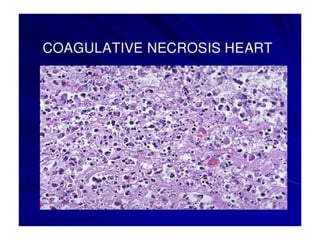
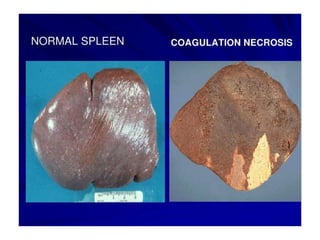
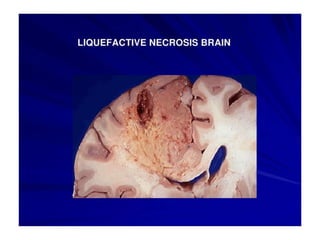
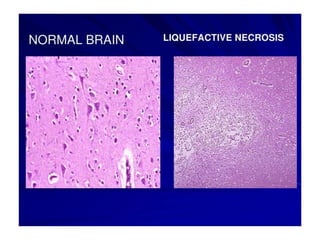
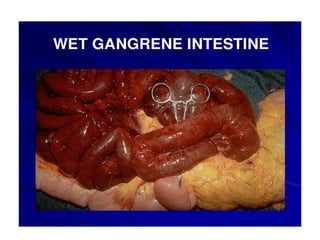
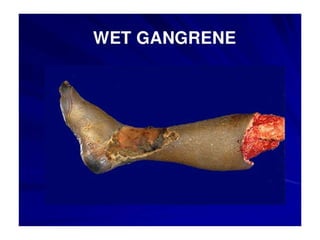
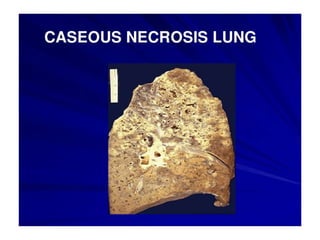
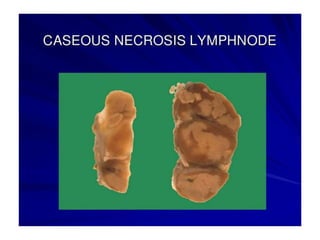
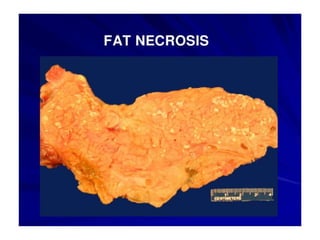
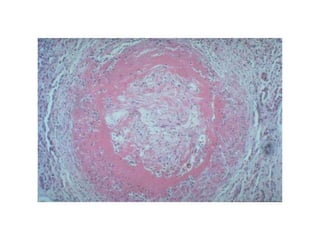
There are two main types of cell death: necrosis and apoptosis. Necrosis is accidental cell death due to external factors like trauma or toxins. It is characterized by cellular contents leaking out and causing inflammation. Apoptosis is programmed cell death that occurs as part of normal development and tissue homeostasis. It is triggered through internal signals or external death ligands binding to receptors. This activates a caspase cascade that breaks down the cell in a controlled, non-inflammatory way. Dysregulation of apoptosis can lead to cancer, autoimmune disease, or neurodegeneration.





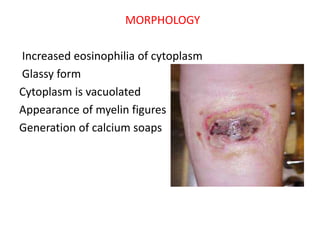













![Chemical and toxic agents (e.g. pharmaceutical drugs,
acids, bases) react with the skin leading to skin loss
and eventually necrosis. Treatment involves
identification and discontinuation of the harmful
agent, followed by treatment of the wound, including
prevention of infection and possibly the use of
immunosuppressive therapies such as anti-
inflammatory drugs or immunosuppressants.[15] In the
example of a snake bite, the use of anti-venom halts
the spread of toxins whilst receiving antibiotics to
impede infection.[16]](https://image.slidesharecdn.com/paathology-150110011810-conversion-gate01/85/Pathology-33-320.jpg)