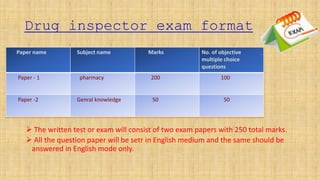
A drug inspector is responsible for monitoring and ensuring the safety, quality, and effectiveness of drugs from production to sale. To become a drug inspector, candidates must have a pharmacy or pharmaceutical science degree, 18 months of relevant work experience, and pass a written exam consisting of two papers testing knowledge of pharmacy and general knowledge. Drug inspectors have the power to inspect any premises or records involved in drug manufacturing, sample and test drugs, inspect licenses, and cancel licenses of businesses found to have quality or standards issues. The role requires skills in discipline, patience, self-confidence, and keeping updated in the pharmaceutical field.