This document discusses the role of NQO1, an NAD(P)H-dependent oxidoreductase, in pancreatic beta cells. The study found that NQO1 protects beta cells from oxidative stress by reducing quinone-dependent reactive oxygen species production. It also found that NQO1 lowers the NADH-to-NAD+ ratio in beta cells, indicating it modulates cellular redox status. Overexpression of NQO1 decreased hydrogen peroxide levels while knockout of NQO1 increased hydrogen peroxide levels in beta cells exposed to oxidative stress. Therefore, NQO1 enhances beta cell health and metabolism by protecting from oxidative damage and regulating redox cycling.



![followed by centrifugation at 12 kg for 5 min at
Then the level of released hydrogen peroxide
4 C. Equal concentrations of cell lysate
(H2O2) was quantified using Amplex
protein were tested for NQO1 activity, which
Red/horseradish peroxidase. Fluorescence (540
was quantified by the decrease in absorbance of
excitation, 595 emission) was monitored using
dichlorophenolindophenol (DPI) (600 nm) over
a SpectraMax M5 multi-mode microplate
a period of one minute. The difference in
reader (Molecular Devices, Sunnyvale, CA).
activity in the absence and presence of
This was evaluated with both rodent islets and
dicoumarol (20 µM) are expressed as NQO1
INS-1 832/13 cells. The rodent islets were
activity. Figure 2
isolated from normal mice and the global knock
out utilizing the corresponding procedures.
NQO1 over-expression in INS-1 832/13 cells.
Adenoviral-mediated over-expression resulted
in the increase of NQO1 protein (adapted
Results
from[10]) and enzyme activity, measured as the
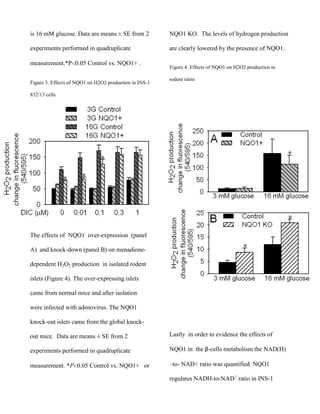
The effects of NQO1 over-expression
reduction of DCPIP
(dichlorophenolindophenol)
on menadione-dependent hydrogen peroxide
production in INS-1 832/13 cells were
measured and analyzed (Figure 3). Dicoumarol
(DIC), an inhibitor of NQO1, blocks NQO1
Control
NQO1+
inhibitory action on redox cycling and H2O2
production. On the first column with no
addition of Dicoumarol NQO1’s protection can
be clearly seen. Under high glucose conditions
NQO1 lowers statistically hydrogen peroxide
production. Legend: 3G is 3 mM glucose, 16](https://image.slidesharecdn.com/paperdmzbbnqo1-140114174618-phpapp01/85/Paper-dmzbb-nqo1-5-320.jpg)