Pulmonary artery banding (PAB) is a palliative surgical technique for congenital heart defects, primarily used to reduce excessive pulmonary blood flow and protect the pulmonary vasculature. Although its usage has declined due to more definitive surgical interventions, PAB remains important for specific patients, especially those with transposition of the great arteries or significant left-to-right shunting. The procedure involves placing a band around the pulmonary artery to manage blood flow and prevent heart failure, with careful consideration of patient selection, anatomical factors, and postoperative care.

![History of the Procedure
• The first description of pulmonary artery banding (PAB) in the
literature was a report by Muller and Dammann at the University of
California, Los Angeles (UCLA) in 1951.
• In this report, Muller and Dammann described palliation by the
"creation of pulmonary stenosis" in a 5-month-old infant who had a
large ventricular septal defect (VSD) and pulmonary overcirculation.
• Following this report, multiple studies were published demonstrating
the effectiveness of this technique in infants with congestive heart
failure (CHF) caused by large VSDs, complex lesions (eg,
atrioventricular canal [AVC] defects), and tricuspid atresia.
• Although the use of PAB has declined, it remains an essential
technique for comprehensive surgical treatment in patients with
congenital heart disease. PAB is a palliative but not a curative
surgical procedure.](https://image.slidesharecdn.com/pabandingnew-1803131814071-240924062810-d8779a5b/75/PA-banding-basic-and-surgical-and-complications-4-2048.jpg)








![Contraindications
• Patients who have single ventricle defects in which the
aorta arises from an outflow chamber (eg, double inlet left
ventricle [LV], tricuspid atresia with transposition of the
great arteries [TGA]) have the potential for development of
significant subaortic obstruction.
• Pulmonary artery banding (PAB) is contraindicated in the
presence of such obstruction and in patients who are at
high risk for such obstruction.
• The ventricular hypertrophy that develops in response to
PAB may cause rapid progression of subaortic obstruction
leading to a combination of both ventricles having outflow
tract obstruction and progressive hypertrophy.](https://image.slidesharecdn.com/pabandingnew-1803131814071-240924062810-d8779a5b/75/PA-banding-basic-and-surgical-and-complications-16-2048.jpg)





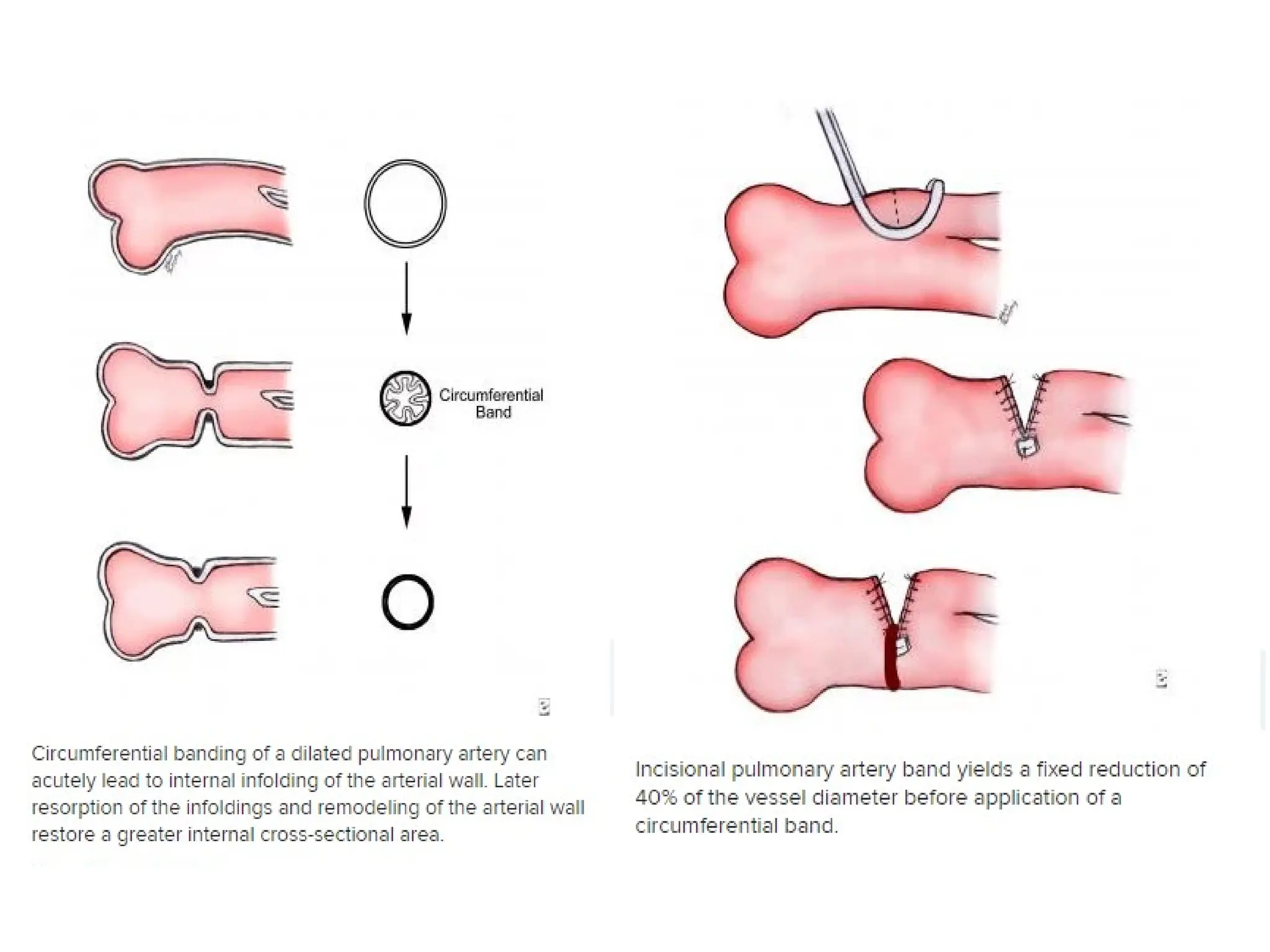
![• The MPA and aorta are exposed, and the band is
prepared for placement.
• The estimated band circumference is marked on the
umbilical tape with fine sutures according to the Trusler
formula.
• PAB circumference in patients with noncyanotic
nonmixing lesions (eg, ventricular septal defect [VSD]) is
20 mm + 1 mm/kg body weight. For patients with mixing
lesions (eg, D-transposition of the great arteries [TGA]
with VSD), the formula is 24 mm + 1 mm/kg body weight.
In patients with single ventricles in whom the Fontan
procedure is planned, an intermediate circumference of
22 mm + 1 mm/kg body weight is preferred.](https://image.slidesharecdn.com/pabandingnew-1803131814071-240924062810-d8779a5b/75/PA-banding-basic-and-surgical-and-complications-23-2048.jpg)





![• An adequate atrial communication should be confirmed
preoperatively in patients with single ventricle physiology,
including transposition with VSD complexes. A balloon
atrial septostomy is useful in these situations.
• Kotani and colleagues measured intraoperative aortic
blood flow using a Transonic flow probe and found that
aortic blood flow increased by approximately 40% after
successful PAB. Their data suggested that higher pre-PAB
Qp/Qs (pulmonary [Qs]-systemic [Qp] blood flow ratio)
predicted a higher percentage increase in aortic flow.
• Three patients with less than a 20% increase in aortic
blood flow died, required re-PAB, or developed
ventricular dysfunction.](https://image.slidesharecdn.com/pabandingnew-1803131814071-240924062810-d8779a5b/75/PA-banding-basic-and-surgical-and-complications-31-2048.jpg)

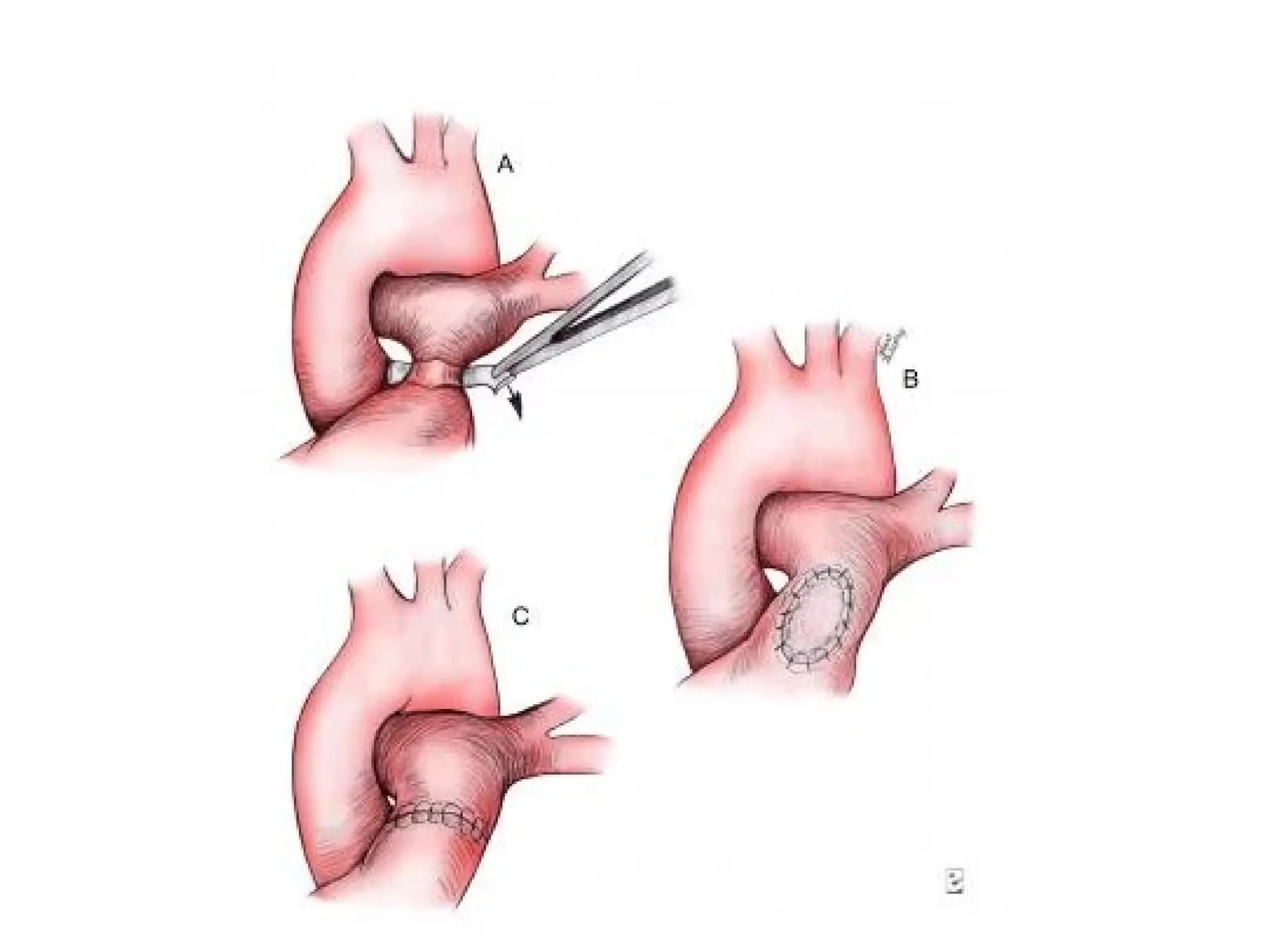
![• PAB takedown is usually performed at the time of the
intracardiac repair through a median sternotomy.
Generally, the repair is completed first and the PAB
removal is performed at the end of the procedure.
• The band is dissected free from surrounding scar tissue
and removed. The area of banding usually remains
stenotic and requires repair.
• This repair can be achieved by resection and end-to-end
anastomosis of the proximal and distal MPA or by
vertical incision of the MPA followed by pericardial (or
polytetrafluoroethylene [PTFE]) patch repair of the
arteriotomy
• The repair must ensure relief of any branch PA stenosis
that may exist as a consequence of the PAB.](https://image.slidesharecdn.com/pabandingnew-1803131814071-240924062810-d8779a5b/75/PA-banding-basic-and-surgical-and-complications-33-2048.jpg)