Oxidative phosphorylation and photophosphorylation are the two main mechanisms by which organisms generate ATP. In oxidative phosphorylation, electrons are passed through an electron transport chain in mitochondria to reduce oxygen to water, pumping protons across the inner mitochondrial membrane. The resulting proton gradient is used by ATP synthase to phosphorylate ADP to ATP. Photophosphorylation uses sunlight to drive electron transport and proton pumping across thylakoid membranes in chloroplasts to similarly synthesize ATP. Both mechanisms conserve the energy of electron transport as a proton gradient that is then used to power ATP synthesis, demonstrating the fundamental similarity between these critical energy conversion processes.
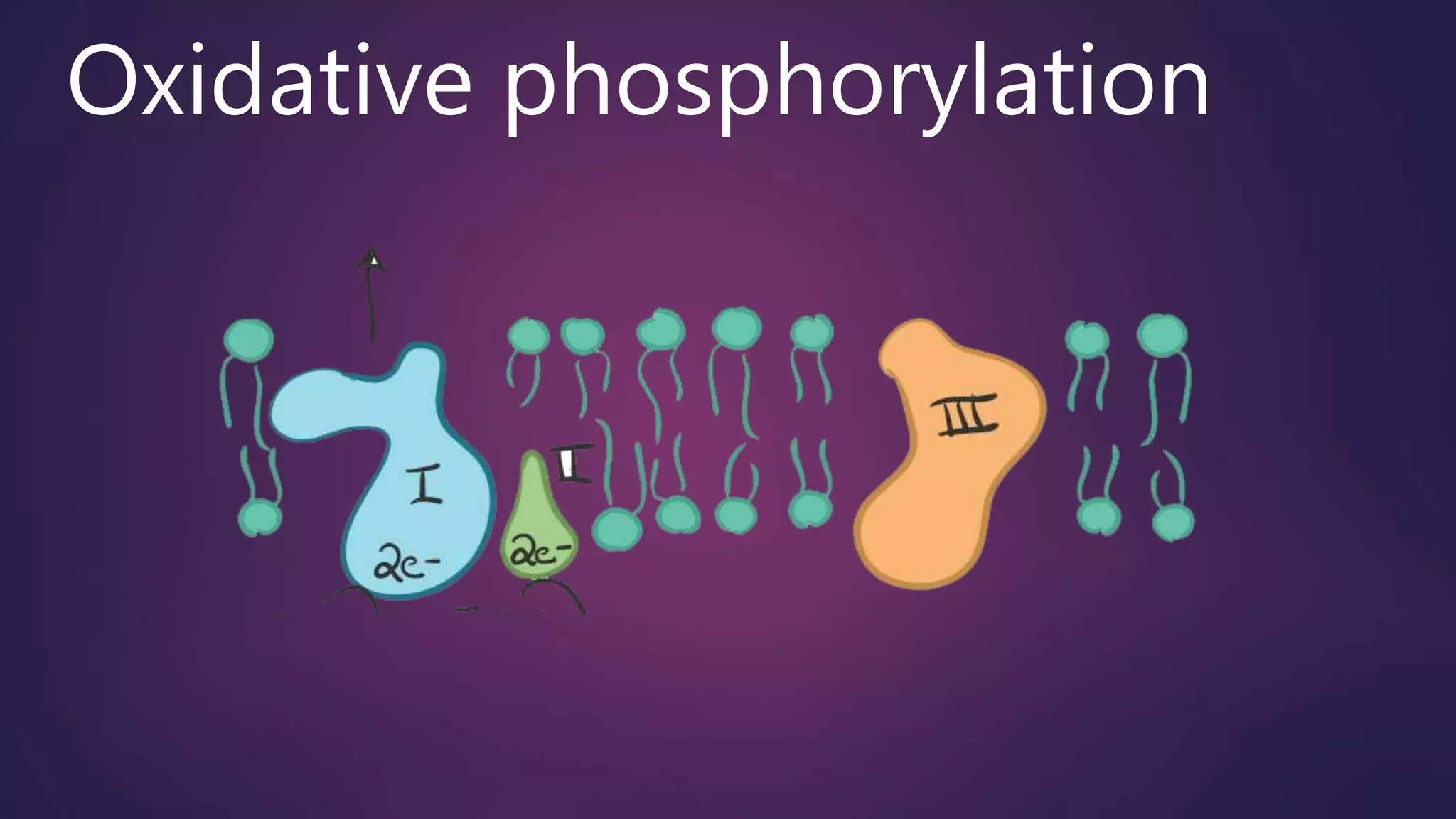





![NADH carries electrons from catabolic reactions to their point of entry into the respiratory chain, the
NADH dehydrogenase complex described below. NADPH generally supplies electrons to anabolic
reactions. Cells maintain separate pools of NADPH and NADH, with different redox potentials.
This is accomplished by holding the ratios of [reduced form]/[oxidized form] relatively high for NADPH
and relatively low for NADH. Neither NADH nor NADPH can cross the inner mitochondrial membrane,
but the electrons they carry can be shuttled across indirectly,
Flavoproteins contain a very tightly, sometimes covalently, bound flavin nucleotide, either FMN or FAD.
The oxidized flavin nucleotide can accept either one electron (yielding the semiquinone form) or two
(yielding FADH2 or FMNH2).
Electron transfer occurs because the flavoprotein has a higher reduction potential than the compound
oxidized. The standard reduction potential of a flavin nucleotide, unlike that of NAD or NADP, depends
on the protein with which it is associated.
Local interactions with functional groups in the protein distort the electron orbitals in the flavin ring,
changing the relative stabilities of oxidized and reduced forms.](https://image.slidesharecdn.com/oxidativephosphorylation-201228030440/75/Oxidative-phosphorylation-7-2048.jpg)













![The intracellular concentration of ADP is one measure of the energy status of cells. Another, related
measure is the mass-action ratio of the ATP-ADP system, [ATP]/([ADP][Pi ]). Normally this ratio is very
high, so the ATP-ADP system is almost fully phosphorylated. When the rate of some energy-requiring
process (protein synthesis, for example) increases, the rate of breakdown of ATP to ADP and Pi
increases, lowering the mass-action ratio.
With more ADP available for oxidative phosphorylation, the rate of respiration increases, causing
regeneration of ATP. This continues until the mass-action ratio returns to its normal high level, at
which point respiration slows again. The rate of oxidation of cellular fuels is regulated with such
sensitivity and precision that the [ATP]/([ADP][Pi ]) ratio fluctuates only slightly in most tissues, even
during extreme variations in energy demand. In short, ATP is formed only as fast as it is used in
energy-requiring cellular activities.](https://image.slidesharecdn.com/oxidativephosphorylation-201228030440/75/Oxidative-phosphorylation-21-2048.jpg)






![SUMMARY for Regulation of Oxidative Phosphorylation
Oxidative phosphorylation is regulated by cellular energy demands. The intracellular [ADP] and the
mass-action ratio [ATP]/([ADP][Pi ]) are measures of a cell’s energy status.
In ischemic (oxygen-deprived) cells, a protein inhibitor blocks ATP hydrolysis by the ATP synthase
operating in reverse, preventing a drastic drop in [ATP].
In brown fat, which is specialized for the production of metabolic heat, electron transfer is
uncoupled from ATP synthesis and the energy of fatty acid oxidation is dissipated as heat.
ATP and ADP concentrations set the rate of electron transfer through the respiratory chain via a
series of interlocking controls on respiration, glycolysis, and the citric acid cycle](https://image.slidesharecdn.com/oxidativephosphorylation-201228030440/75/Oxidative-phosphorylation-28-2048.jpg)

