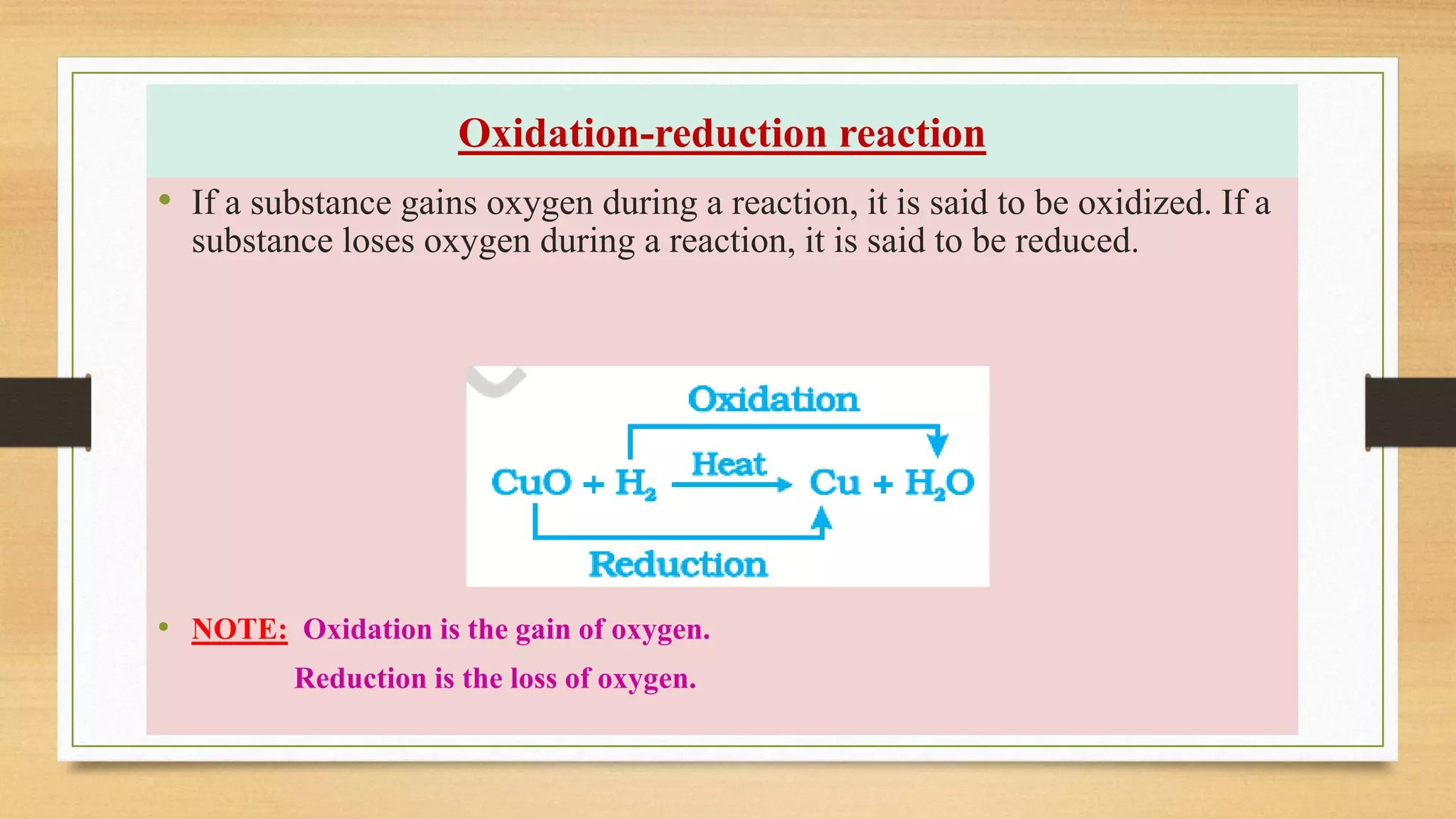
This document discusses chemical reactions and equations. It defines oxidation as a gain of oxygen and reduction as a loss of oxygen during a reaction. It explains that corrosion occurs when the surface of a metal is attacked by air, moisture, or other substances, such as rusting of iron. It also discusses rancidity, which is the oxidation of fats and oils over time that changes their color, smell, and taste.