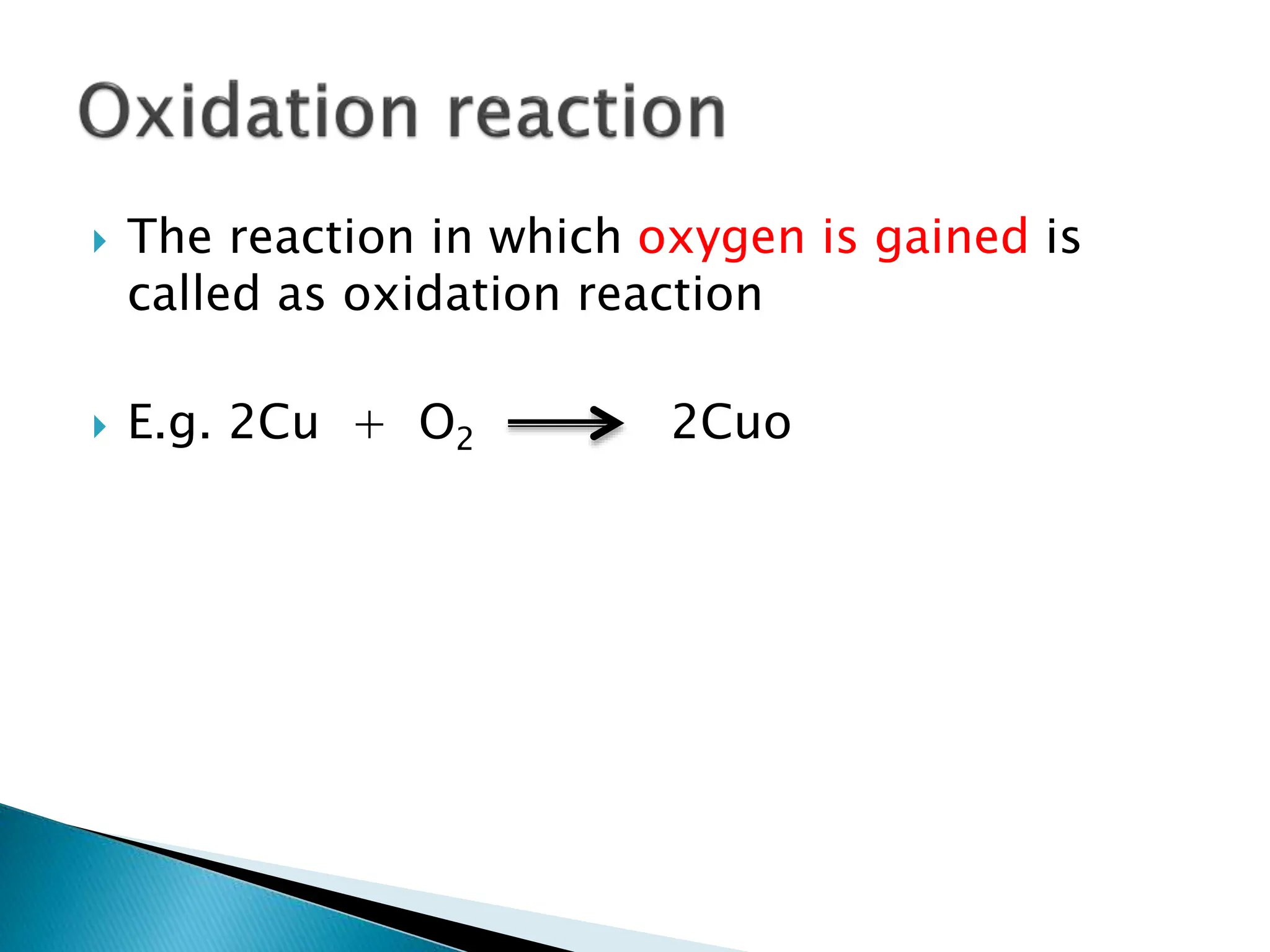
The document explains various types of chemical changes, including combination, decomposition, displacement, oxidation, reduction, redox reactions, and their corresponding equations. It also discusses the process of balancing chemical equations and provides examples for each type of reaction, alongside concepts of exothermic and endothermic reactions, corrosion, and rancidity. Additionally, it outlines prevention methods for corrosion and rancidity.