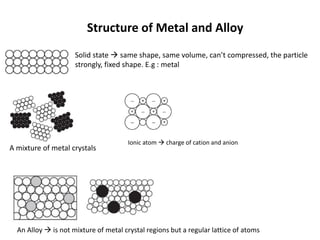
This document discusses metallic elements and alloys. It notes that metals are found on the left side of the periodic table and have properties like conductivity, malleability, and reactivity. Common reactions of metals include reacting with water to form hydroxides, acids to form salts, and oxygen to form oxides. Alloys are mixtures of metals or metals with other elements designed to have specific properties. Examples given are brass, stainless steel, and aluminum alloys. Metals have many important uses due to their properties, like iron in construction, aluminum in aircraft, and copper in wiring.