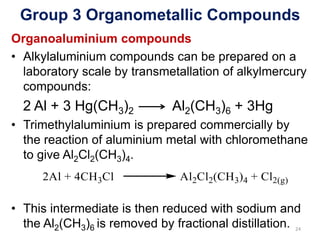
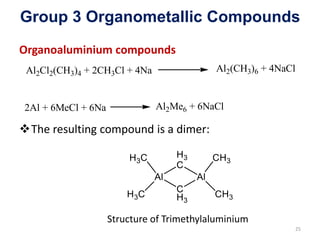
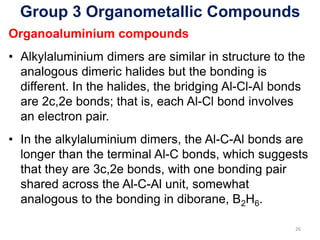
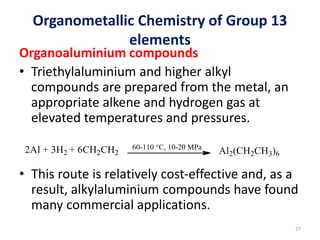
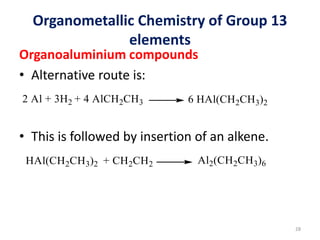
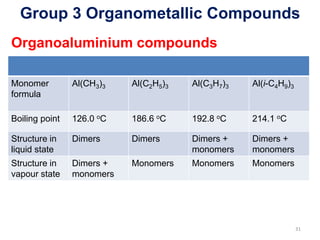
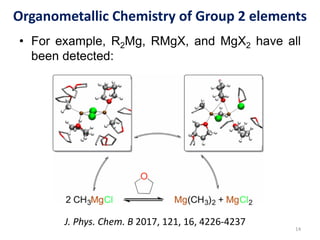
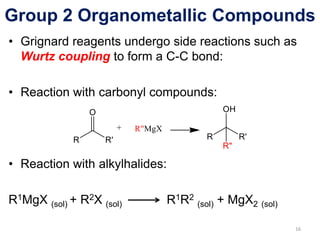
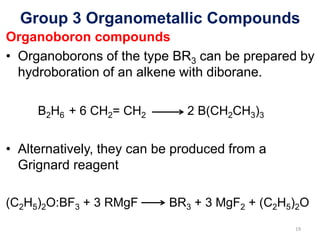
The document provides an overview of organometallic compounds, focusing on those of group 2 and group 3 elements. It discusses various synthesis methods, structures, and properties of organoberyllium and organoboron compounds, emphasizing their applications in organic synthesis and coordination chemistry. Additionally, it highlights the structural variations of organoaluminium compounds and their industrial significance.

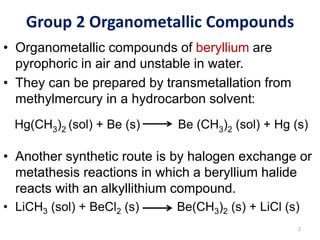

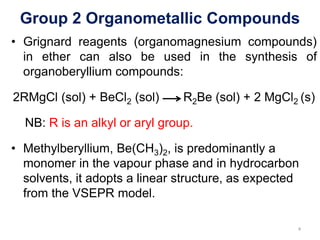
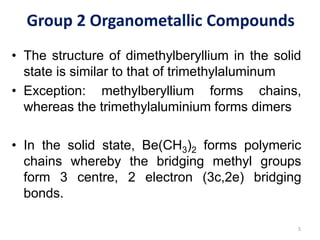
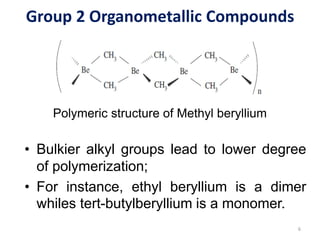

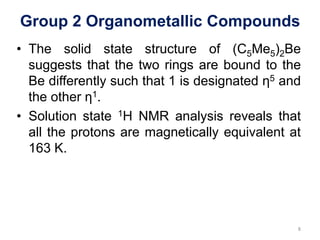
![Group 2 Organometallic Compounds
• Assignment: Read the following article for discussion in
our next lecture:
Synthesis, solid-state structure, and bonding analysis of
the beryllocenes [Be(C5Me4H)2], [Be(C5Me5)2], and
[Be(C5Me5)(C5Me4H)] (DOI: 10.1002/chem.200304876)
Solid state structure of
beryllocene (SC-XRD)
9
Solution state structure
of beryllocene (1H
NMR)](https://image.slidesharecdn.com/chem352-note224-240708110920-dd860da9/85/CHEM-352-Note-inorganic-chemistry-notes-9-320.jpg)







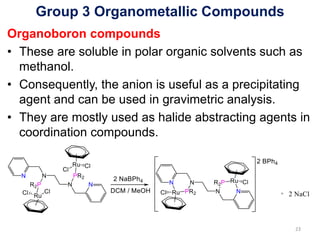
![Group 3 Organometallic Compounds
Organoboron compounds
• An important anion is the tetraphenylborate ion,
[B(C6H5)4]-, more commonly written BPh4
-.
• An analogous to the tetrahydroborate ion, BH4
-.
• The sodium salt can be obtained by a simple
addition reaction:
BPh3 + NaPh Na+[BPh4]-
21](https://image.slidesharecdn.com/chem352-note224-240708110920-dd860da9/85/CHEM-352-Note-inorganic-chemistry-notes-21-320.jpg)
![Group 3 Organometallic Compounds
Organoboron compounds
• The sodium salt of [B(C6H5)4]- is used as a
counterion for cationic coordination
compounds.
• An analogue is sodium tetrakis[3,5-
bis(trifluoromethyl)phenyl]borate, popularly
known as NaBArF.
22](https://image.slidesharecdn.com/chem352-note224-240708110920-dd860da9/85/CHEM-352-Note-inorganic-chemistry-notes-22-320.jpg)