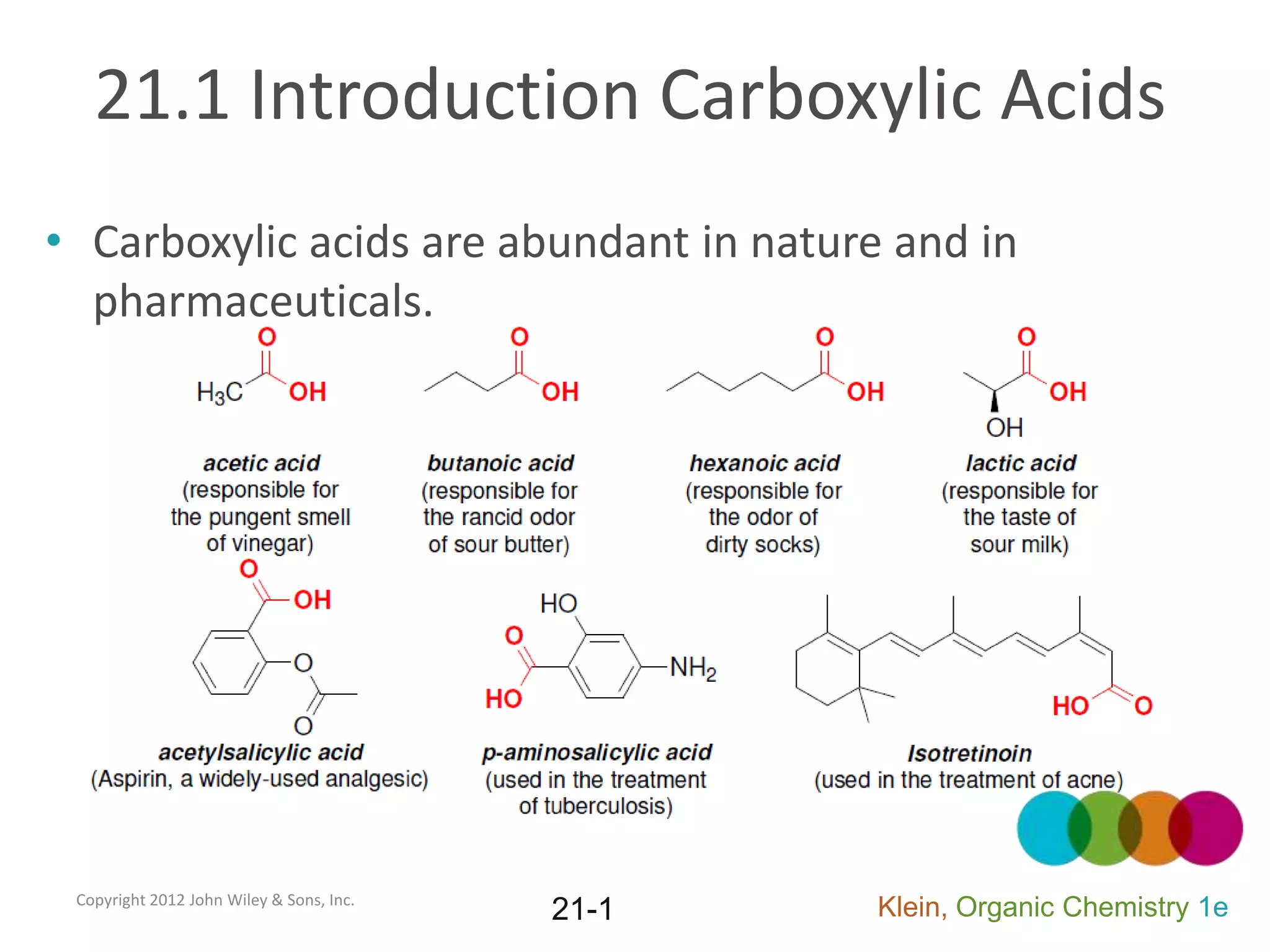
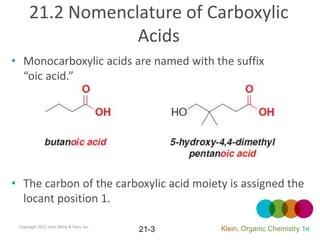
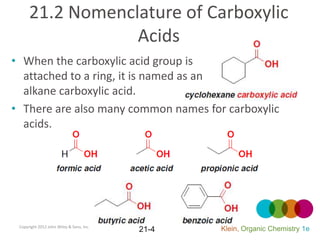
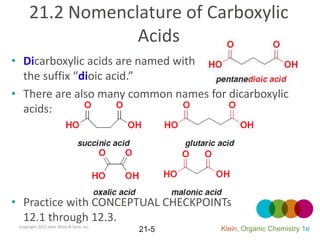
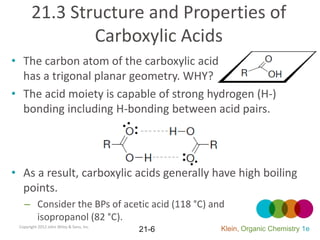
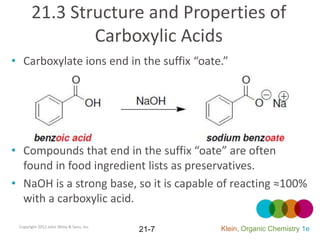
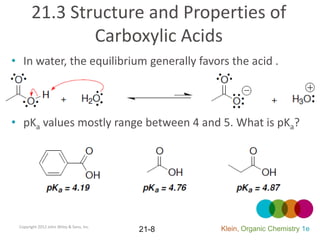
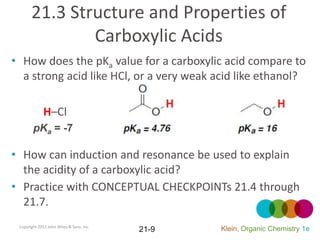
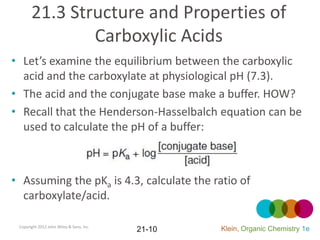
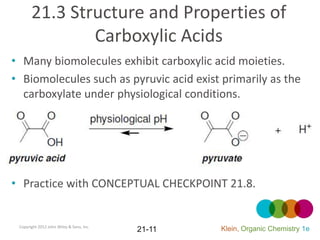
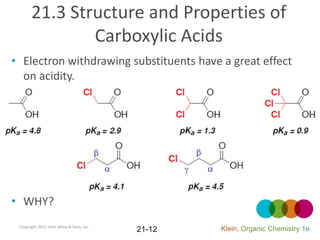
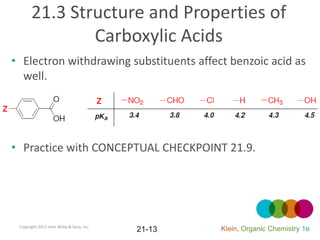
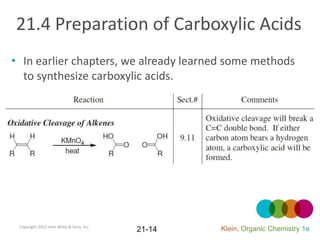
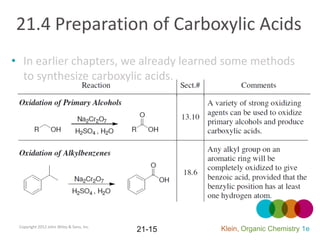
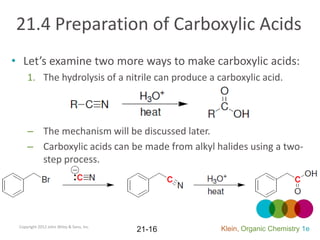
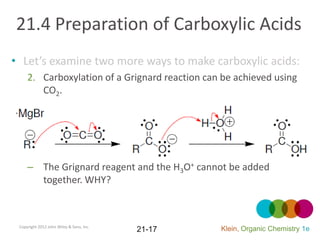
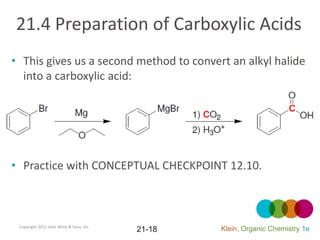
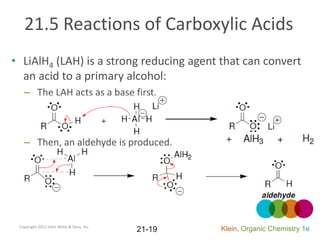
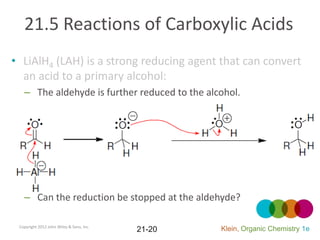
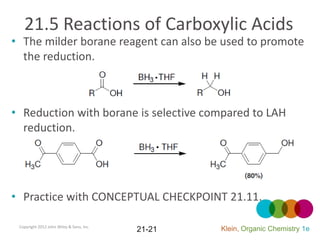
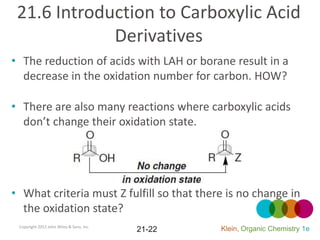
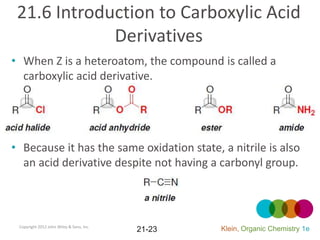
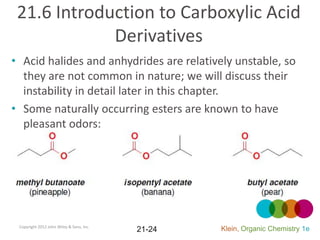
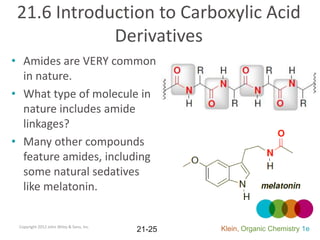
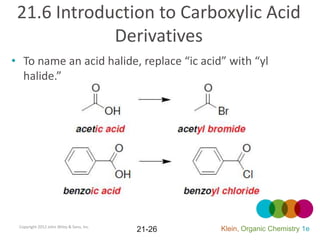
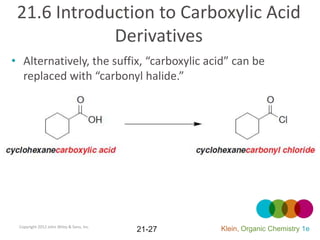
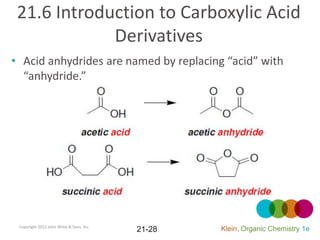
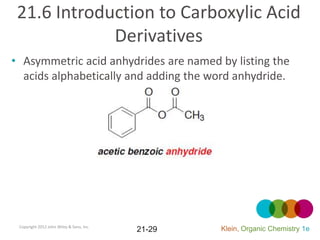
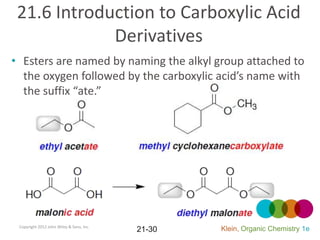
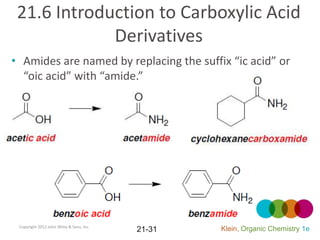
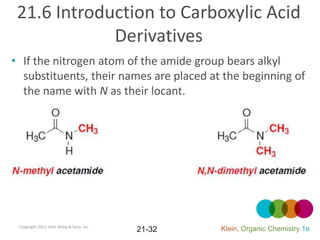
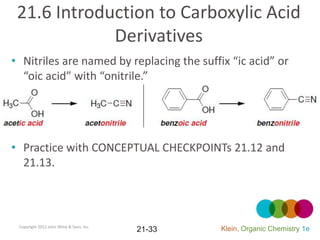
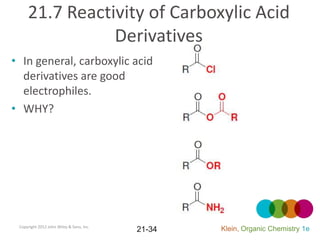
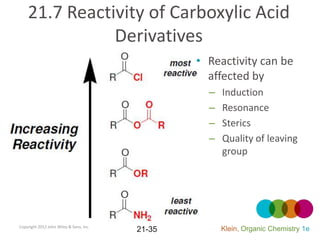
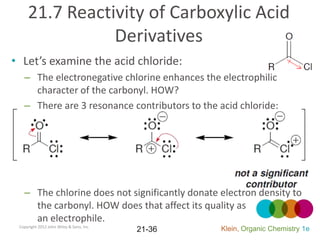
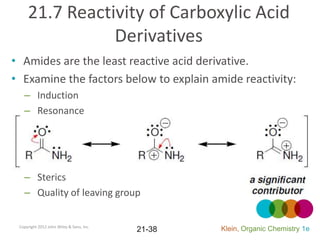
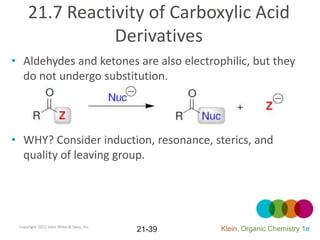
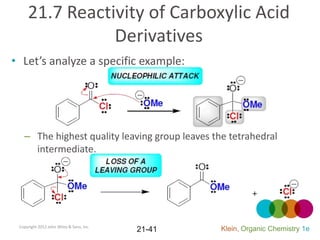
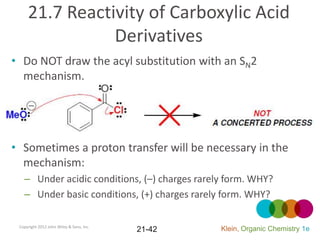
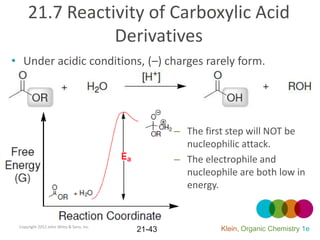
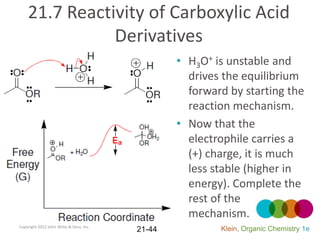
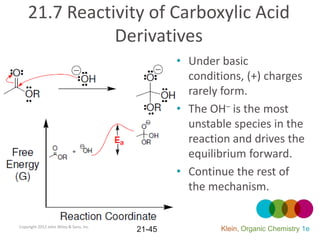
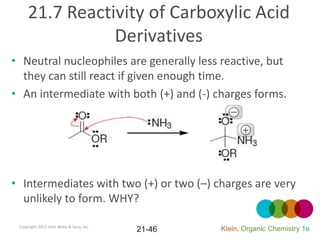
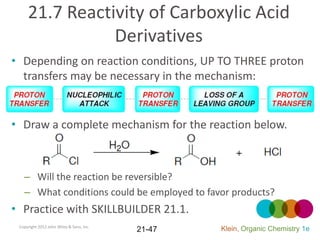
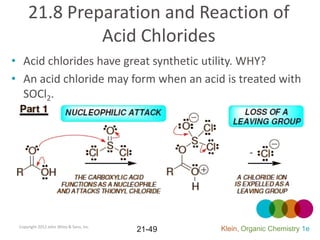
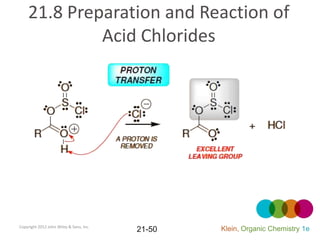
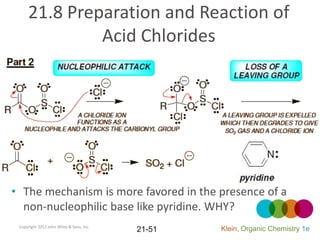
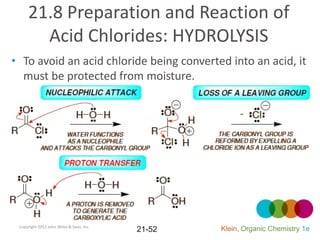
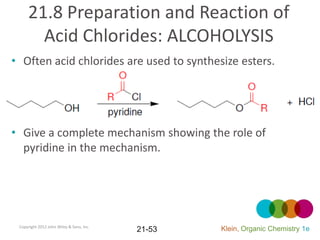
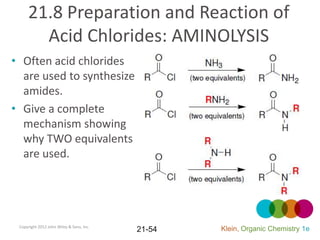
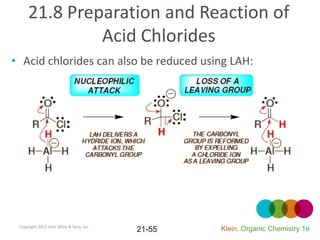
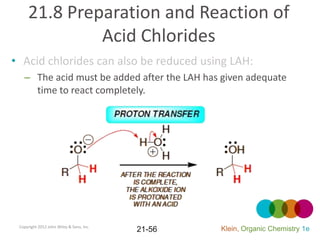
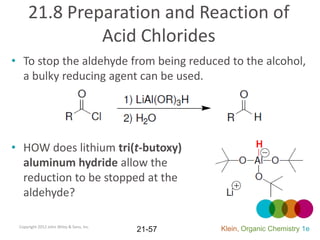
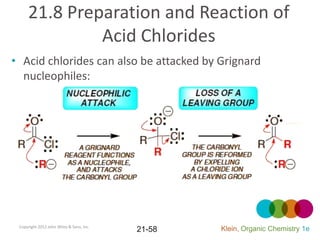
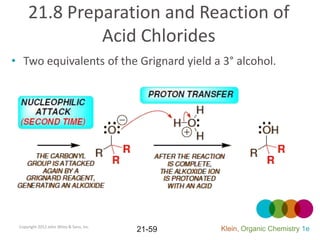
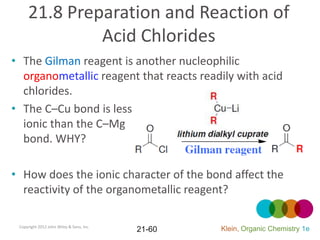
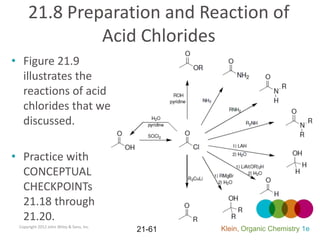
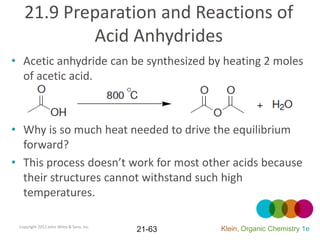
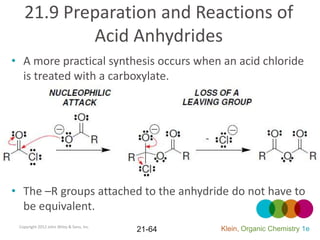
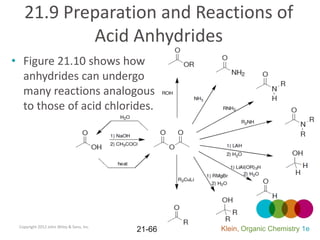
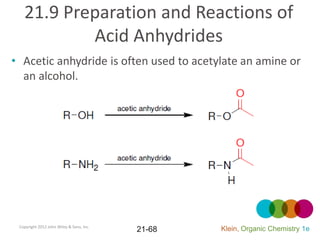
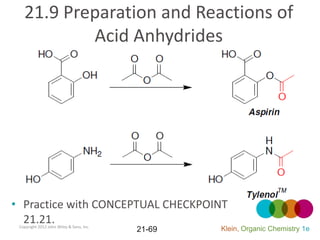
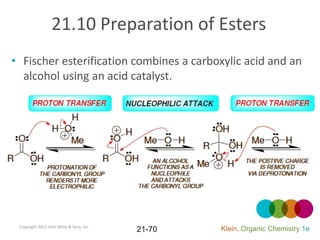
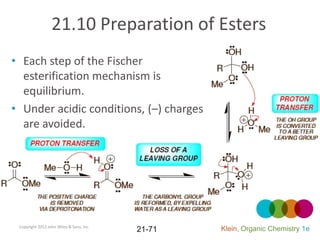
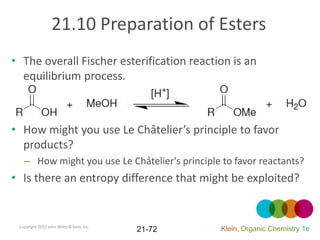
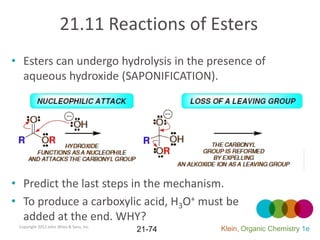
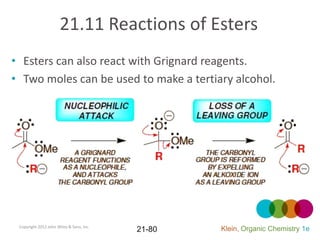
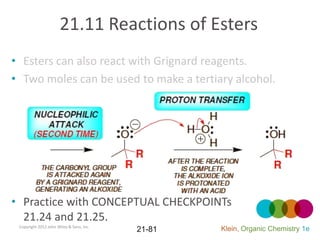
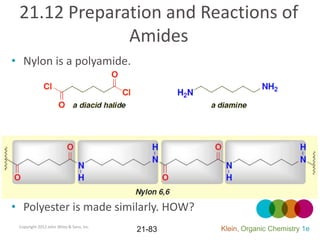
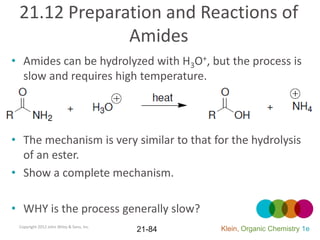
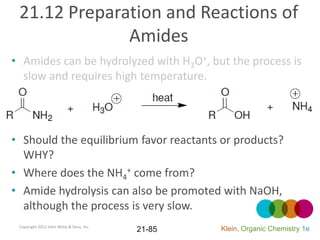
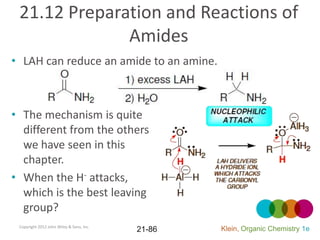
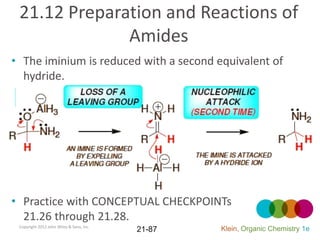
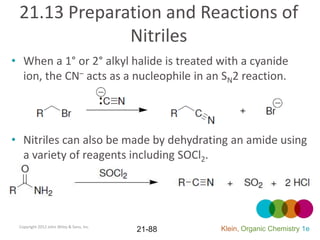
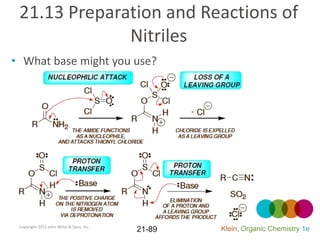
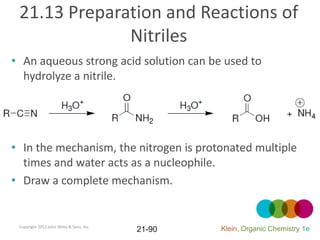
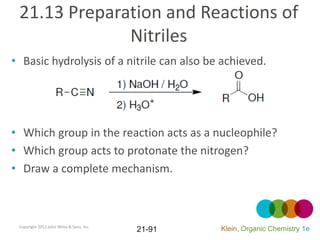
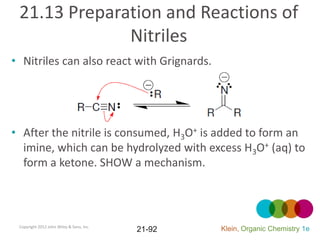
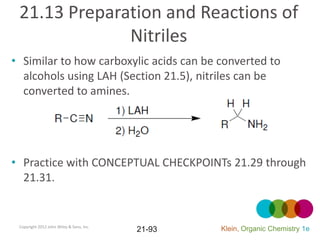
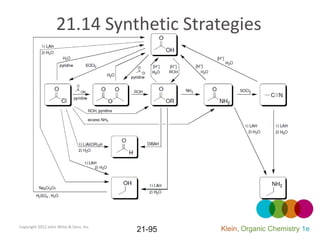
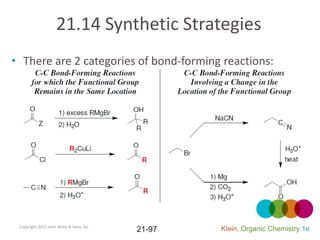
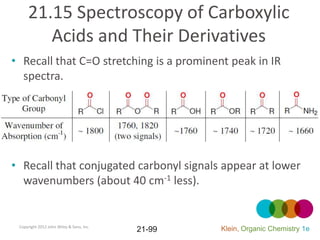
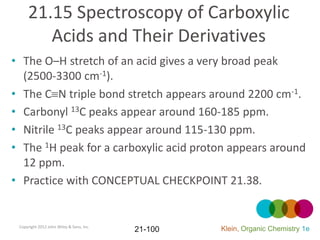
This document provides an introduction to carboxylic acids and their derivatives. It discusses the abundance and uses of carboxylic acids in nature and industry. Key topics covered include the nomenclature of carboxylic acids, the structure and properties that result from the carboxylic acid functional group, such as acidity and hydrogen bonding. Methods of preparing and reacting carboxylic acids are also summarized, along with an introduction to common carboxylic acid derivatives like esters, acid halides, anhydrides, and amides.