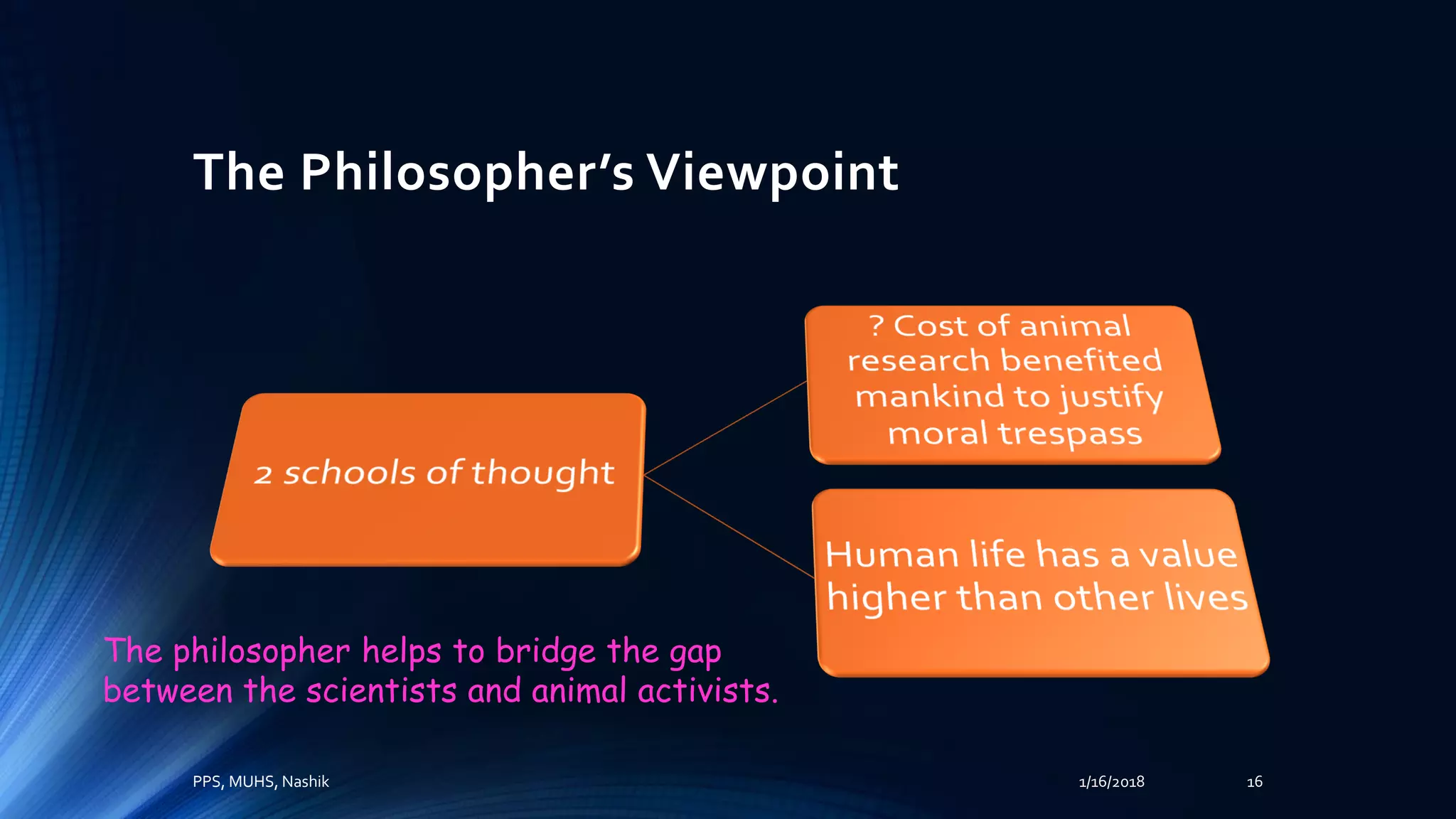
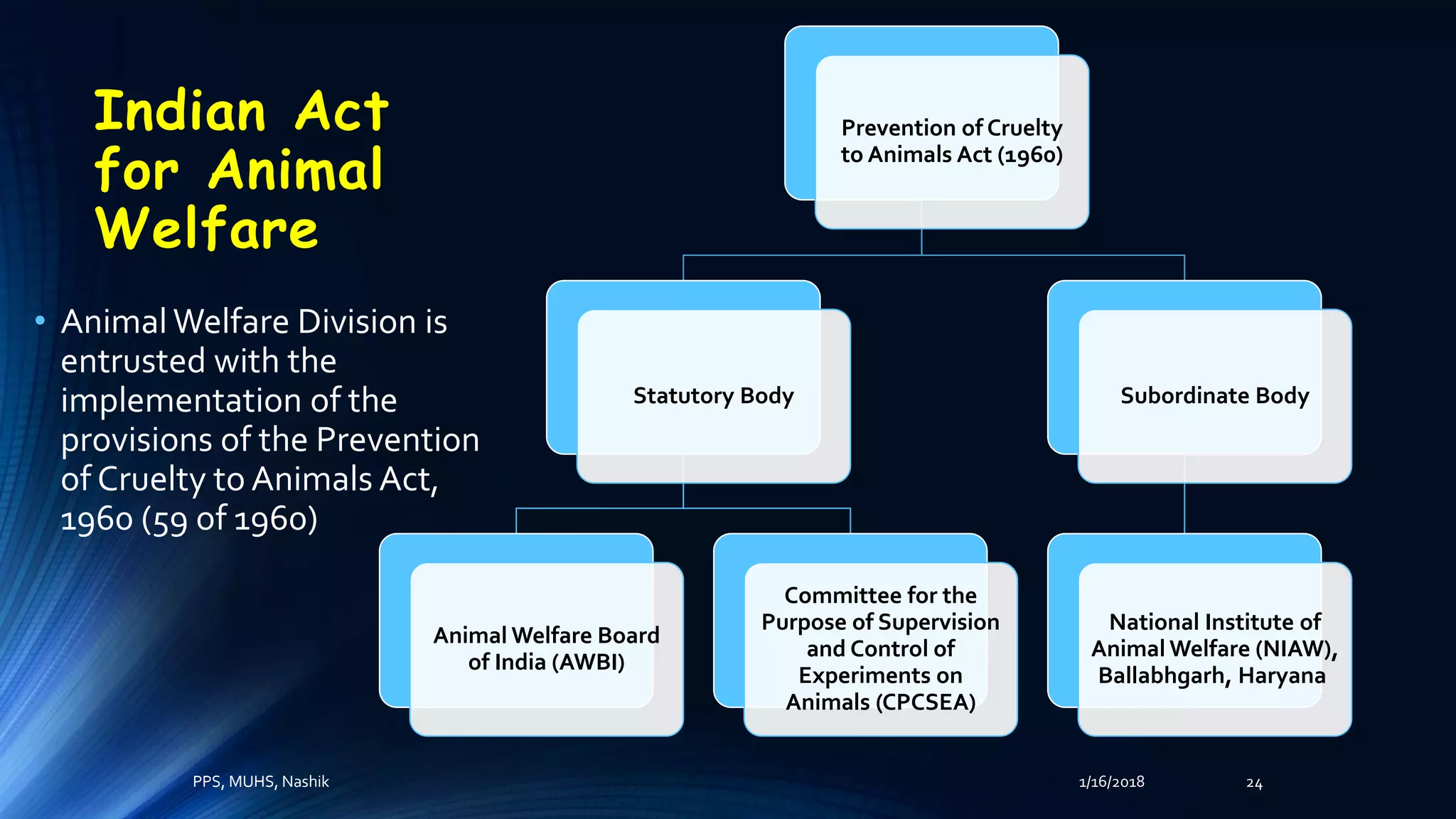
The document discusses the role of laboratory animals in medical research, emphasizing their importance for scientific advancements while also highlighting the ethical concerns surrounding their use. It outlines the differing perspectives of scientists, animal activists, and philosophers on this issue and introduces the principles of humane experimental techniques focusing on the 3Rs: reduction, refinement, and replacement. Additionally, it details regulatory frameworks and the structure of institutional animal ethics committees in India to ensure animal welfare during research.