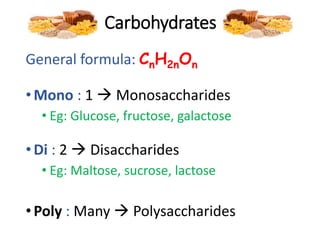
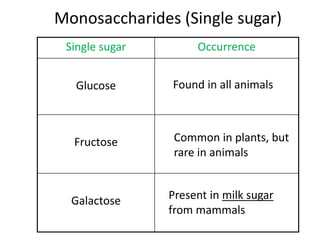
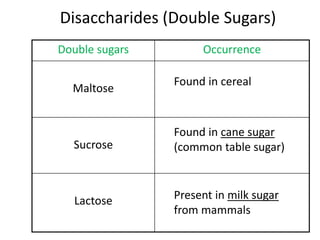
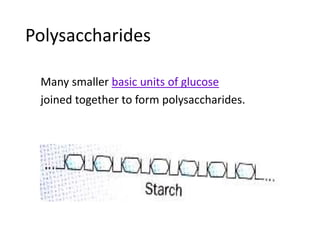
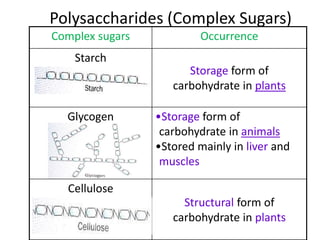
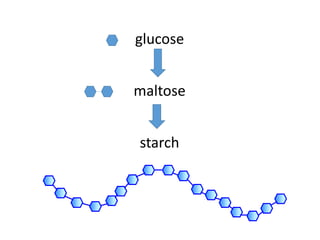
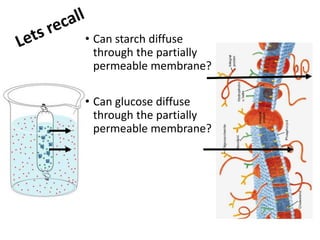
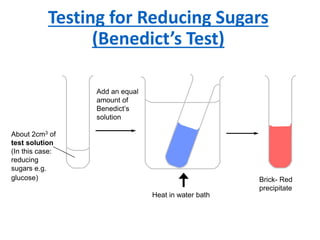
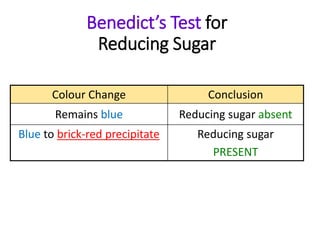
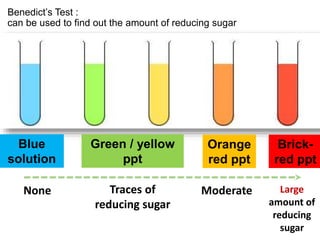
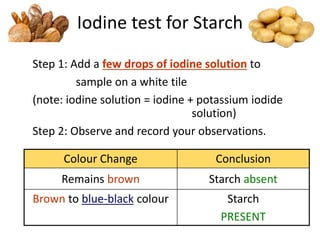
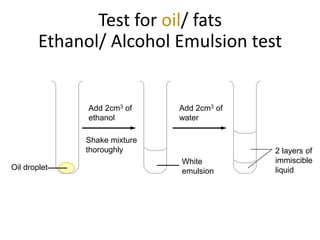
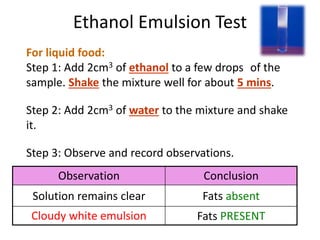
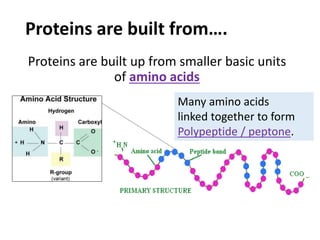
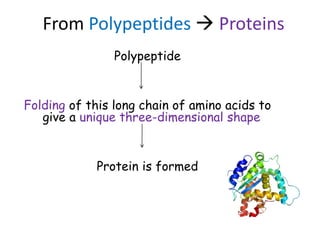
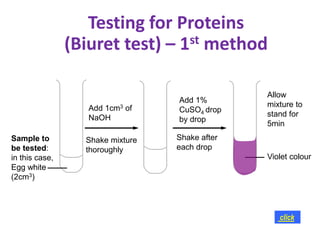
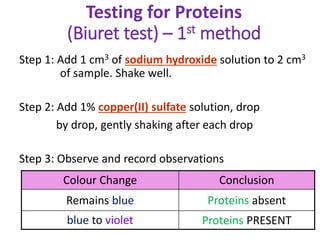
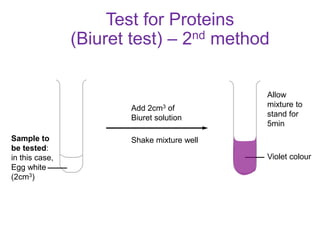
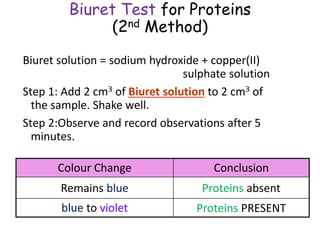
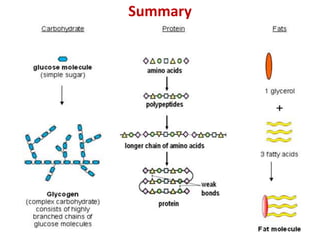
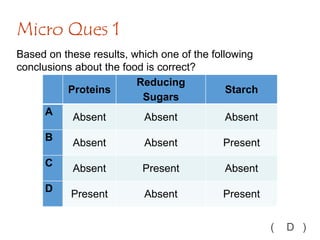
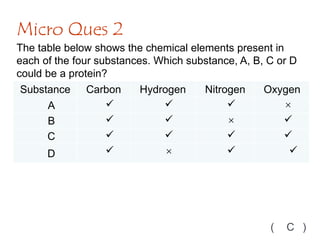
The document discusses different nutrients including carbohydrates, proteins, and fats. It describes the chemical composition and tests used to identify these nutrients. Carbohydrates include monosaccharides like glucose, disaccharides like sucrose, and polysaccharides like starch. Proteins are made of amino acids linked together, while fats contain fatty acids and glycerol. Tests described include Benedict's test for reducing sugars, iodine test for starch, and Biuret test for proteins. The document provides information on the nutrients present in different foods.