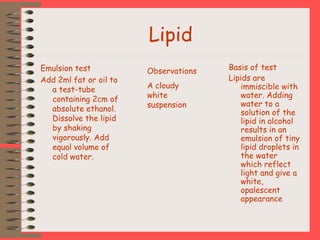
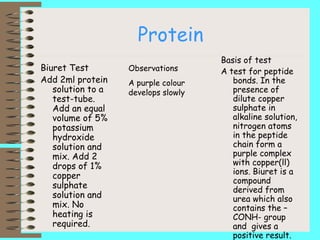
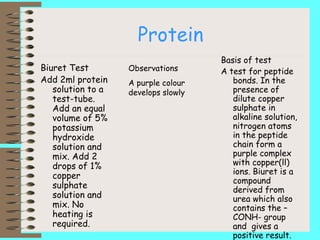
This document describes biochemical tests to identify reducing sugars, non-reducing sugars, starch, lipids, and proteins. It provides details of the procedure, basis, and expected observations for each test. The reducing sugar test uses Benedict's solution and looks for a color change from blue to green to yellow to red. The non-reducing sugar test hydrolyzes sugars like sucrose before using Benedict's solution. The starch test uses iodine and looks for a blue-black color. The lipid test makes an emulsion in water to look for an opaque white appearance. The protein test uses potassium hydroxide and copper sulfate to detect peptide bonds from a purple complex formation.