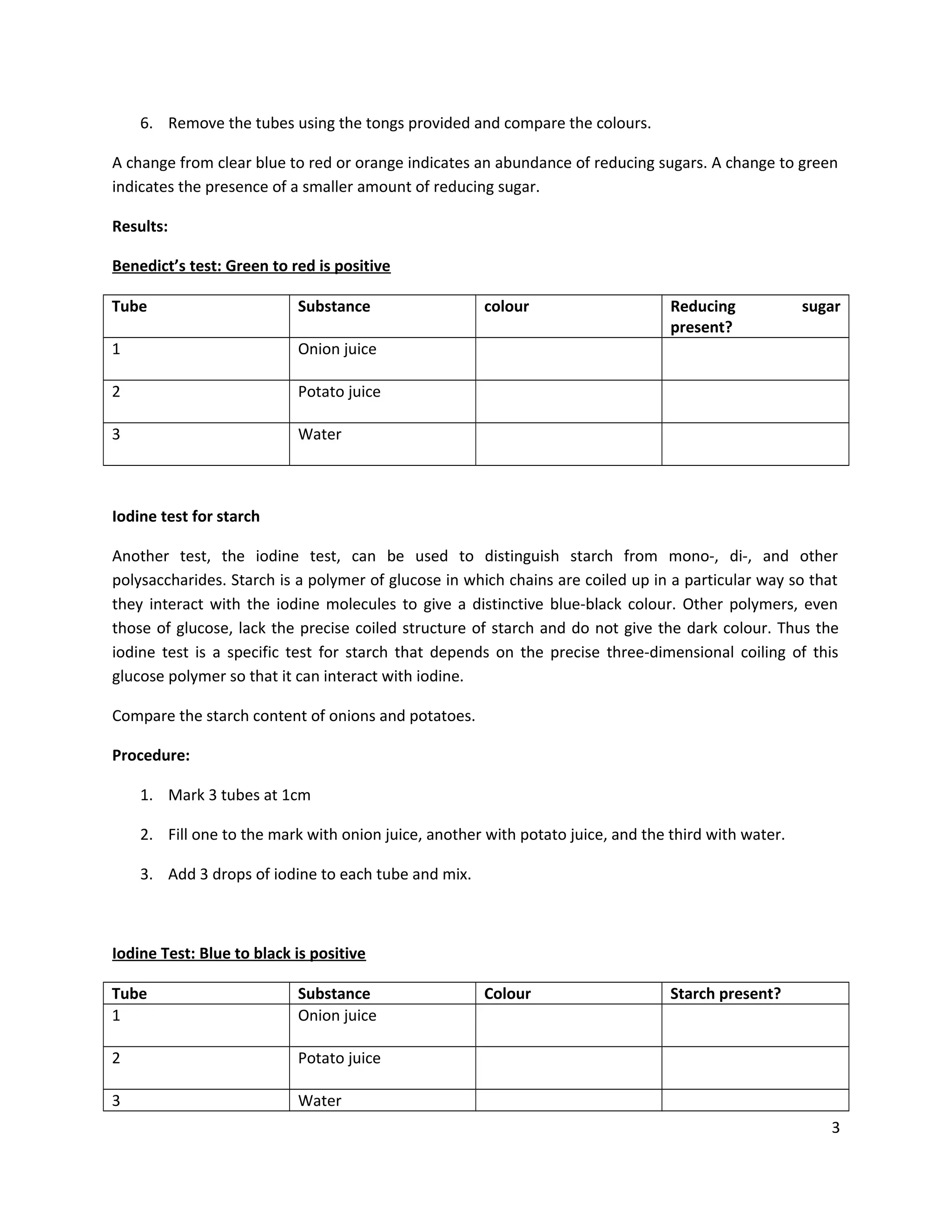
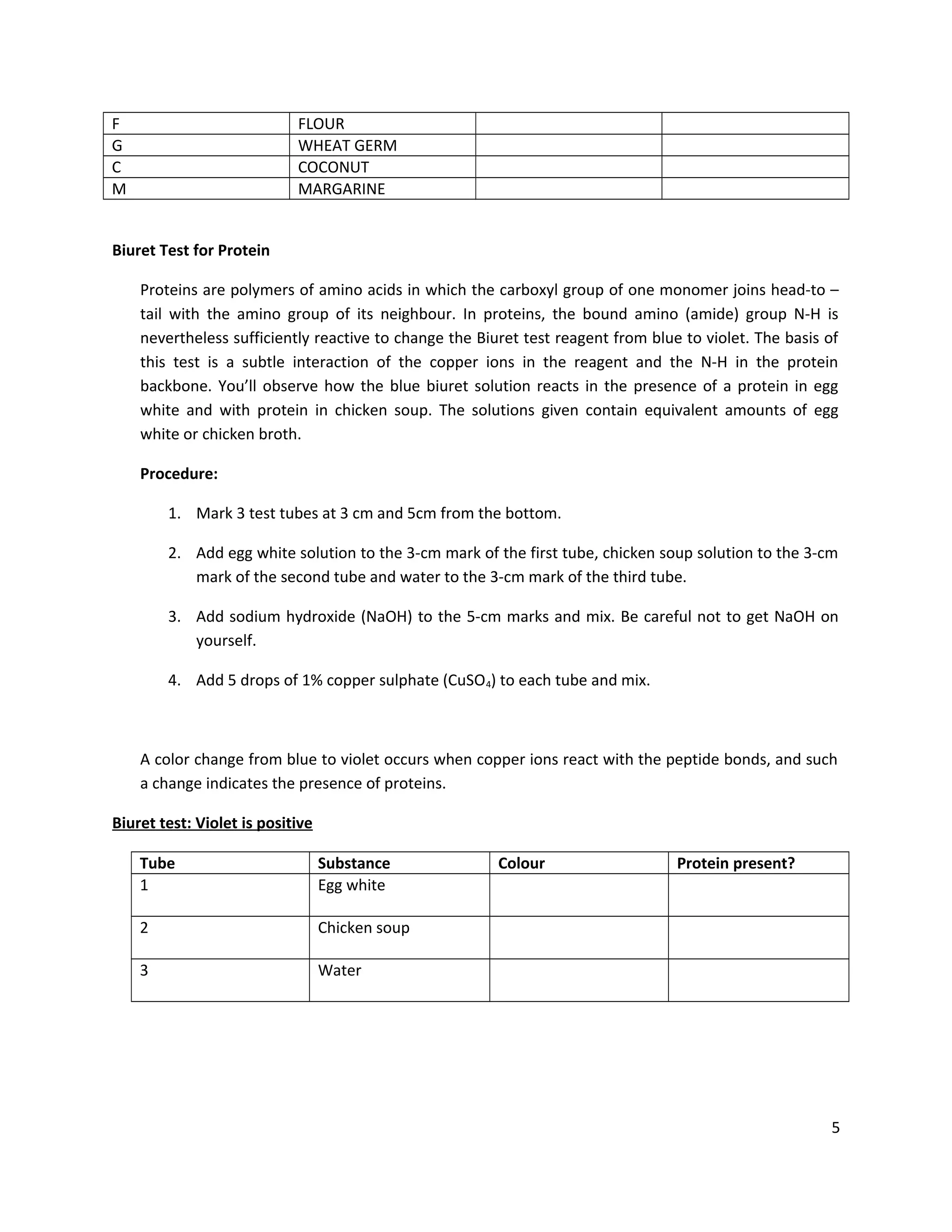
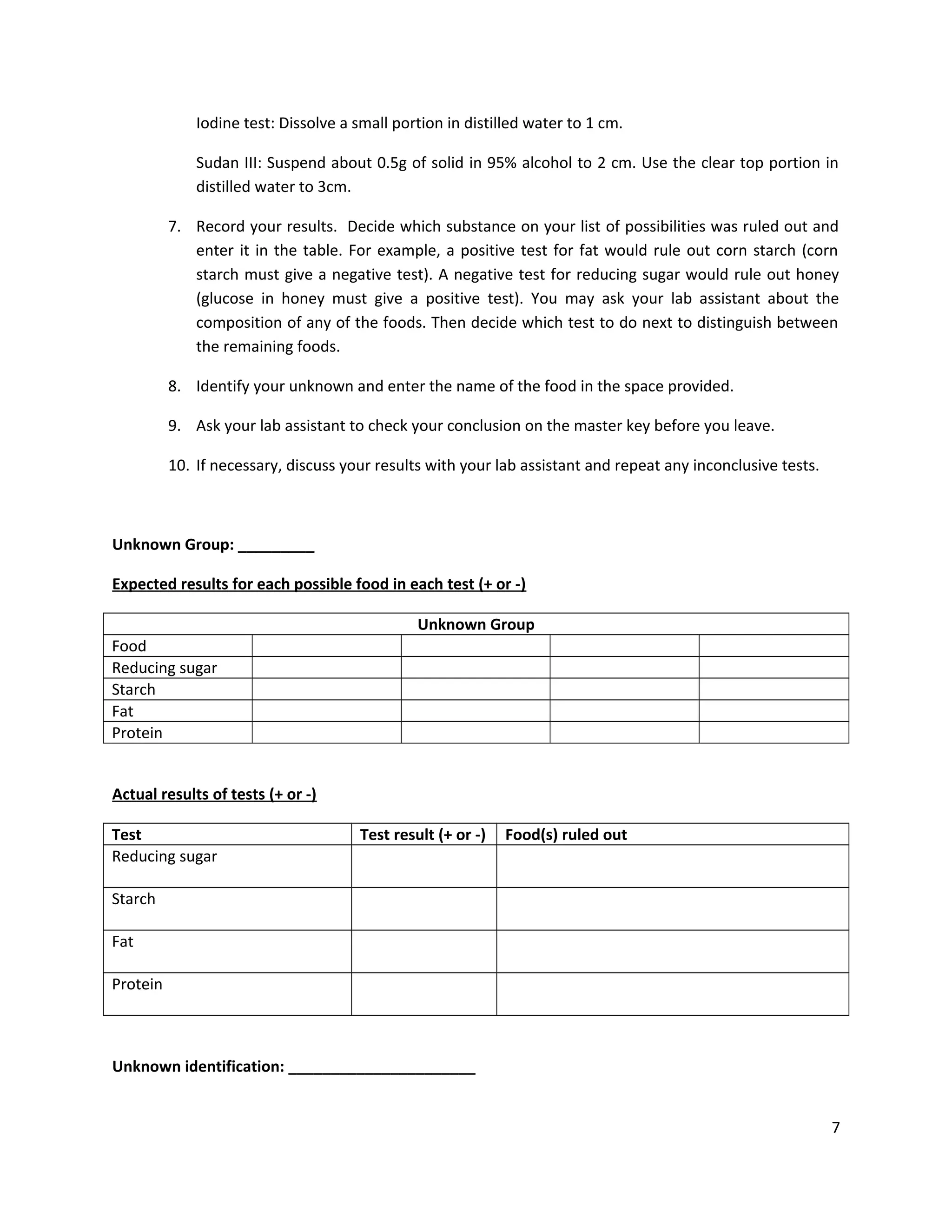
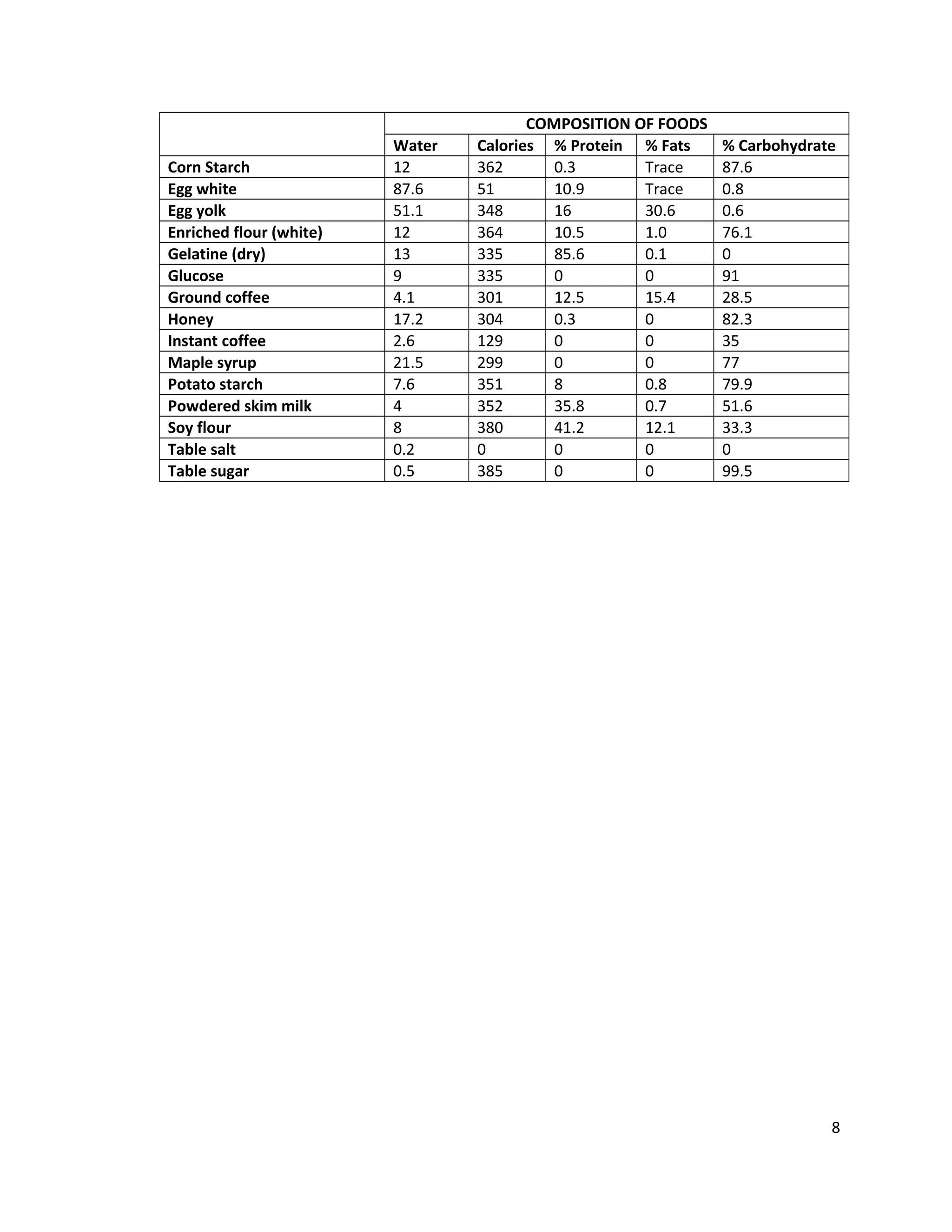
This document provides instructions for students to test unknown biological samples to identify their key components. It begins by defining functional groups and listing the most common elements in living things. The bulk of the document describes how to use specific chemical tests to detect carbohydrates, proteins, fats, and starch. These include Benedict's test for reducing sugars, iodine for starch, Sudan III for fats, and biuret for proteins. Students are given unknown samples assigned to categories of possible foods and asked to predict test results to identify the unknown based on the results of applying these chemical tests.